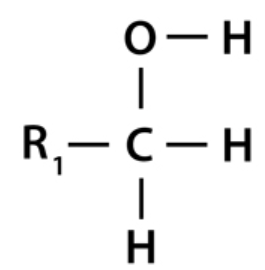
Alcohols are the most well-known chemical compounds due to their many uses and functions in our daily lives. Alcohols are chemical molecules with a hydroxyl group attached to either aryl or an alkyl group (ROH). The hydroxyl atom is bonded to just one R group in primary alcohol. If it contains two R groups, it is classified as secondary alcohol; if it contains three R groups, it is classed as tertiary alcohol. As a wide range of other chemical compounds, alcohols may be aromatic due to the presence of a benzene ring. The most fundamental aromatic alcohol is phenol.
1. Primary alcohols
In primary alcohols, the carbon atom of the hydroxyl group (OH) is bonded to just one alkyl group. Examples include methanol (propanol), ethanol, and other major alcohols. Any alcohol’s principal characteristic has nothing to do with the complexity of this alkyl chain. The primary alcohol is defined by a single connection between a –OH group and an alkyl group.
example:
2. Secondary Alcohols
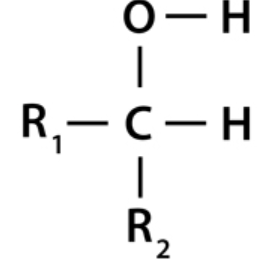
Secondary alcohols contain the carbon atom of the hydroxyl group connected to two alkyl groups on each side. The two alkyl groups present may be the same or different structurally. Secondary alcohol examples include 2 – propanol and 2 – butanol.
example:
3. Tertiary alcohols
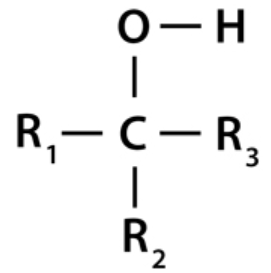
Secondary alcohols contain the carbon atom of the hydroxyl group connected to three alkyl groups on each side. The structure of these alcohols contributes significantly to their physical properties. Alcohols may form hydrogen bonds with their neighbouring atoms due to the presence of the -OH group. Due to the weak bonds formed, alcohols have a higher boiling point than alkanes.
example:
Importance of Alcohols
- Alcohol is a versatile medication that may be utilised for many purposes. A few instances are shown below.
- Alcohol is ingested in beverages containing 30% and 40% ethanol by volume.
- They are blended with a solution of ethylene glycol in water to act as an anti-freezing agent.
- Ethanol, more often referred to as ethanol, is used as an antiseptic.
- Some alcohols, such as methanol, are used as fuel in internal combustion engines.
- Several of them are used in medicine as preservatives for laboratory specimens.
Identification of Alcohol
The determination of primary alcohol, secondary alcohol, and tertiary alcohols may be accomplished in several ways. Alcohol identification may be accomplished in organic chemistry by examining the various characteristics of several alcohol types. The instruments are analysed using various methods, including nuclear magnetic resonance (NMR) and qualitative testing. Alcohol may be identified in the same way as ketones and aldehydes are identified.
Alcohol Detection Technique
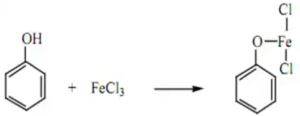
1. Ferric Chloride Test
Iron (III) chloride is one way for differentiating aromatic and aliphatic alcohols. The iron chloride component imparts a reddish-orange hue to the solution. When aromatic alcohol, such as phenol, is present, the chlorine atoms are replaced by the aromatic alcohol owing to the core iron atom’s coordination behaviour changing. As a consequence, the solution becomes purple. The solution retains its vibrant red-orange colour because aliphatic alcohols do not react with iron (III) chloride.
2. Jones test
The Jones test, which employs chromium trioxide as an oxidising agent in the presence of sulfuric acid, is another technique for detecting the presence of alcohol. In the presence of Jones’ reagent, a primary alcohol is first converted to an aldehyde. After that, the primary alcohol is transformed into a carboxylic acid, while the secondary alcohol group is converted to a ketone. The chromium oxidation state is critical in this assay. Chromium is at the oxidation state +6 in Jones’ reagent. The presence of Cr(VI) complexes imparts a brilliant red and orange hue to the reagent.
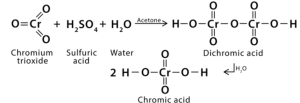
Chromium is reduced chemically from Cr (VI) to oxidation state +3 — Cr (III). To begin, when chromic acid and alcohol acid interact, chromate ester is created. When H2O, a base, cleaves the C-H alcohol link, it forms the carbonyl group, transforming Cr(VI) to Cr (IV). Two electrons are lost from the Cr(IV), while the alcohol’s carbon loses two electrons. As a consequence, the reduction-oxidation phase is referred to as this phase.
Additionally, Cr(IV) participates in future oxidation steps before being reduced to Cr (III). The most abundant forms of chromium (III) are Hexa aqua chromium (III) ions — [Cr(H2O)6]3+ — and Cr(III) complexes with one or more sulphate ions — [Cr(H2O)5(SO4)]+ (III). Each of these complexes contains Cr(III), which imparts their characteristic green colour.
Since tertiary alcohols do not react with chromium, no precipitate forms, maintaining the solution’s orange colour. Consequently, the Jones test may be used to determine the presence of primary, secondary, or tertiary alcohols.
Difference between Primary, Secondary, and Tertiary
- Primary – Alcohols composed of carbon(C) atoms coupled to a hydroxyl (OH) group. These alcohols include a -CH2OH group, with the OH group often located near the carbon chain’s terminal.
- Secondary – Alcohols with a carbon atom and a hydroxyl (OH) group should have a two-carbon bond. These alcohols include a -CHOH group with an OH group in the carbon chain’s midsection.
- Tertiary – Alcohols with a carbon(C) atom bonded to a hydroxyl (OH) group should include direct connections to the three carbon atoms. These alcohols have the -COH group, and the OH is often located at the branched carbon chain junction. Count the number of carbon atoms directly linked to the carbon bonded to the OH group to determine the degree of alcohol.
Conclusion
Now that you have an in-depth understanding of the identification of secondary alcohols. During the course, we learned that these alcohols are considered derivatives of water molecules where one of the hydrogen atoms is replaced by an alkyl group (commonly denoted by the symbol R).
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out