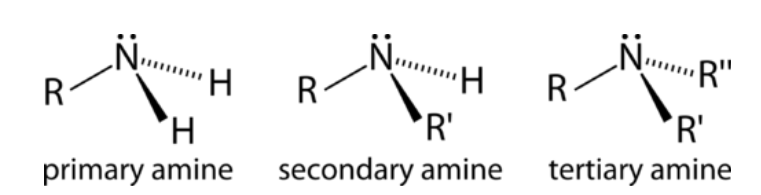
A hydrogen atom from the ammonia molecule is replaced by an alkyl or even an aryl group in amines – organic molecules. In this structure, carbon atoms are firmly linked to nitrogen atoms. It is referred to as a secondary amine when it bonds with two carbon atoms, and a tertiary amine when it bonds with three carbon atoms. The chemical characteristics and physical modifications of primary, secondary, and tertiary amines are all distinct. They are utilised mostly in industrial and commercial settings.
Amines are known for their distinctive features, such as their distinct odours. The smell of rotting eggs or fish is usually the cause of these odours. On the other hand, aromatic amines tend to have a lower density and a stronger ammonia bond than aliphatic amines do. Rubber, dyes, medicines, synthetic resins, and fibres are the most common industrial uses. A number of tests are used to identify primary, secondary, and tertiary amines, respectively.
For the most part, a chemical test known as the Hinsberg test is used to identify several types of amines, including primary, secondary, and tertiary. A Hinsberg reagent reacts with an amine in the presence of an aqueous alkali.
Identifying the Amines type
The number of carbon atoms directly linked to the nitrogen atom determines the classification of amines as primary, secondary, or tertiary. There is only one carbon linked to the nitrogen in primary amines.
In contrast, the nitrogen is joined to two carbons in secondary amines. Finally, the tertiary amines contain three carbon atoms linked to nitrogen via covalent bonds. It appears that all of these amines have been categorised in the same way as alcohols.
On the other hand, alcohols are distinguished by the fact that the OH group is counted as a link to the carbon atom. In the case of amines, the carbons linked to the nitrogen are counted as carbons. Secondary and tertiary amines exhibit a wide range of chemical properties and physical changes that might be observed. Commercial and industrial use is the most common scenario in which they are put to use.
Characteristics of Amines
Regardless of whether an amine is a primary, secondary, or tertiary amine group, they all tend to have their own unique characteristics, such as distinct odours. The smell of rotting eggs or fish is often compared to these scents. It is a well-known fact that aliphatic amines are less dense than water and have stronger ammonia connections than aromatic amines, which are less dense than water.
Primary and secondary amines are commonly employed to produce colours, rubber, synthetic fibres, resins, and medicines. Primary, secondary, and tertiary amines are all identified using different methods. The Hinsberg test is one of the most popular. Hinsberg’s response is the name given to this test’s outcome. Let’s learn more about the Hinsberg test and Hinsberg’s response.
Hinsberg Test
The primary, secondary, and tertiary amines can be identified using the Hinsberg test. It is necessary to thoroughly shake the supplied amine and the Hinsberg reagent with aqueous alkalies, such as NaOH or KOH, to perform this test. An acidic solution of sodium hydroxide mixed with Benzenesulfonyl chloride will then be introduced to the substrate. Sulphonamide salts can be formed by primary amines.
The sulphonamide of this primary amine is precipitated as a result of this salt’s acidification. Similar reactions will take place to generate an insoluble sulphonamide with secondary amines. However, sulfonamide will not reactivate the tertiary amine because it is insoluble. This particular insoluble amine is transformed into a soluble ammonium salt by the addition of dilute acid. All three forms of amines are distinguished in this manner.
For this reason, you should be aware that while the Hinsberg test described above is fairly reliable, it is not infallible. In other words, the Hinsberg test for amine identification only works if the reaction speed, temperature, concentration, and solubility of the amines in question are taken into account.
As a result, the Hinsberg test is an excellent tool for determining the presence of primary, secondary, and tertiary amines in various samples. Insecticides and rocket propellants are only two examples of the various uses for amines. Because of this, amines are used in a wide variety of industries outside of their typical chemical applications.
Conclusion
When a Hinsberg reagent reacts with a primary amine, an amide known as N-Ethylbenzenesulphonyl amide is commonly generated, whereas the reagent employed is known as benzene sulfonyl chloride. As a result of the nitrogen compound’s hydrogen attachment, this is a highly acidic solution. Consequently, it dissolves in alkali.
Therefore, it is possible to use the Hinsberg method to identify primary, secondarily, and tertiary-amine compounds. Amines can be utilised for a wide range of applications, including medicine and photography. Rocket propellants and pesticides can also be made using this amine. In addition to their typical chemical applications, amines are used in a wide range of industrial applications. For military purposes, it is utilised to make synthetic fibers, which are used to make Kevlar, a crucial component in bulletproof vests and helmets for soldiers’ protection in wars.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out