Amines
When an organic compound with one or more hydrogen atoms of the ammonia molecule is replaced with an alkyl or even an aryl group, those compounds are classified as amines. These are carbon atoms which are bonded to the nitrogen atom and their bond is pretty strong. Amines are classified into different categories namely, primary, secondary, and tertiary depending on the range of carbons which can be bonded immediately to the nitrogen atom. Primary amines have simply one carbon that is bonded to the nitrogen. Secondary amines, on the alternative hand, have carbons which can be bonded to the nitrogen. And lastly, the tertiary amines have 3 carbons which are bonded to the nitrogen. The system of classification of most of these amines is superficially comparable in the manner we’ve got categorized alcohols. However, the essential distinction is that during alcohols we remember bonds to the carbon that carry the OH group. In the case of amines, we count the carbons which can be bonded to the nitrogen. All of those number one, secondary and tertiary amine display various chemical properties and feature observable physical changes. They have made their use commonly withinside the industrial and commercial programs. Today, we can learn about the identity of number one amine, secondary amine, tertiary amine, the number one amine, secondary aromatic amine and tertiary amine definition, and the primary, secondary, tertiary amine formula.
Primary Amines, Secondary Amines and Tertiary Amines
Amines are in addition divided into three exceptional types; which can be:
- Primary amine: Only one of the hydrogen atoms in the ammonia molecule has been changed in primary amines. That means the principal amine’s formula will be RNH2 , where “R” is an alkyl group.
Example:
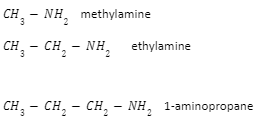
- Secondary amine: Two of the hydrogens in an ammonia molecule have been replaced by hydrocarbon groups in a secondary amine. At this level, you’re more likely to run across simple ones with both hydrocarbon groups being alkyl and both being the same.
Example:
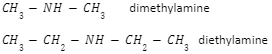
3. Tertiary amine: All of the hydrogens in an ammonia molecule have been replaced by hydrocarbon groups in a tertiary amine. Again, you’re more likely to run across simple ones with all three hydrocarbon groups being alkyl and all three being the same.
Example: trimethylamine
Once it bonds with 2 carbon atoms it is referred to as a secondary amine and whilst it bonds with three, it is referred to as a tertiary amine. Primary, Secondary and Tertiary amines all display exceptional chemical properties and still have physical, observable changes. They are especially utilised in commercial and industrial programs.
Amines normally have specially distinct properties about them, including their characteristic odours. These odours normally that of rotting eggs or fishes. Aliphatic amines are amines which are much less dense than water and are normally more potent bonds of ammonia than fragrant amines. The main industrial programs are in making rubber, dyes, prescribed drugs and artificial resins and fibres. Certain assessments are done for the identity of primary amines, secondary amines, and tertiary amines. One of the most famous assessments is the Hinsberg test and the response made out of this test is referred to as the Hinsberg response.
Properties of Amines
Physical Properties
- Lower aliphatic amines are naturally gaseous. They have a fishy odour.
- At normal temperature, primary amines with three or four carbon atoms are liquids, whereas higher amines are solids.
- Aniline and other arylamines are colourless in general. However, they get discoloured when left out in the open owing to air oxidation.
- Lower aliphatic amines have the ability to create hydrogen bonds with water molecules. As a result, such amines are water soluble.
Chemical Properties
- Because of the presence of an unshared electron pair, amines function as nucleophiles.
- Amines are reactive due to the difference in electronegativity between nitrogen and hydrogen atoms, as well as the existence of an unshared pair of electrons above the nitrogen atom.
- The reactivity of primary, secondary, and tertiary amines change due on the amount of hydrogen atoms bound to nitrogen.
Hinsberg test
A chemical test at this maximum typically used for the identity of number one, secondary and tertiary amines is referred to as the Hinsberg test at. An amine withinside the presence of an aqueous alkali interacts with a Hinsberg reagent. Thus, that is what is supposed because the Hinsberg test at. After the response, the following observations are observed: Getting a primary amine to react with a Hinsberg reagent normally ends in an amide being formed, referred to as N-ethylbenzenesulphonyl amide, while the reagent used is referred to as benzene sulfonyl chloride. This is strongly acidic because the hydrogen is connected as part of the nitrogen compound. Thus, this answer is likewise soluble in alkali. Thus, the Hinsberg test is powerful for the identity of primary amines, secondary amines, and tertiary amines. Amines are normally used for quite a few exceptional purposes including quite a few exceptional drugs and photographs. Apart from this, the amine is likewise used for the synthesis of rocket propellants and insecticides. Thus, amines have an entire host of various makes use of withinside the enterprise together with their conventional chemical makes use of. It is likewise utilised in heavy-responsibility military features including the advent of artificial fibres, used in the manufacturing of Kevlar, that’s the important thing in growing helmets and bulletproof vests for the safety of soldiers in warfare.
Hoffman Mustard Oil Reaction
In the Hofmann mustard oil response of number one amines, the black precipitate is because of HgS. It is a test of number one amine. Primary amine offers alkyl isothiocyanate having a mustard oil like smell. Secondary amine doesn’t show Hofmann’s mustard oil response.
Conclusion
Amines are classified according to the number of carbon atoms bonded directly to the nitrogen atom. A primary (1°) amine has one alkyl (or aryl) group on the nitrogen atom, a secondary (2°) amine has two, and a tertiary (3°) amine has three .
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




