The greater stability of carbon-carbon double bonds as the degree of substitution increases has been attributed to hyperconjugation. Early hyperconjugation research was carried out by George Kistiakowsky’s research group.
Definition
The hyperconjugation effect is a permanent effect in which electrons of an alkyl group’s C-H bond are directly connected to an unsaturated system atom or an atom with an unshared p orbital.
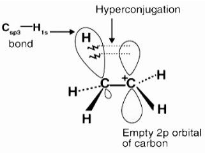
One of the three C-H bonds of the methyl group can align in the plane of the empty p orbital, and the electrons comprising the C-H bond in this plane can then be delocalized into the empty p orbital, as seen in the diagram above.
The hyperconjugation additionally stabilises the carbocation by assisting in the distribution of positive charges. As a result, we may say that the more alkyl groups connected to a positively charged carbon atom, the more hyperconjugation interaction and carbonation stabilisation there is. On the basis of hyperconjugation, the relative stability is given as,
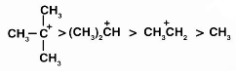
Stabilisation of 1,3-Butadiyne and 1,3-Butadiene
Kistiakowsky was the first to investigate the conjugation of 1,3-butadiene, discovering a conjugative contribution of 3.5 kcal/mol based on an energy comparison of hydrogenation between conjugated and unconjugated counterparts. Rogers stated that the conjugation stabilisation of 1,3-butadiyne was zero, since the difference of ΔhydH between first and second hydrogenation was zero, using the approach first utilised by Kistiakowsky. The hydrogenation temperatures (ΔhydH) were calculated using the computational G3(MP2) quantum chemistry approach.
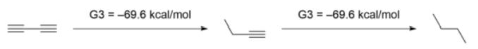
Another group led by Houk claimed that Rogers and Kistiakowsky approaches were ineffective because temperatures of hydrogenation comparisons take into account not only conjugation effects but also

other structural and electrical differences.
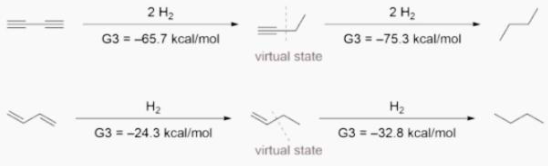
Ab initio calculations yielded -70.6 kcal/mol for the first hydrogenation and -70.4 kcal/mol for the second hydrogenation, respectively, confirming Rogers’ findings. They did, however, interpret the data differently because of the hyperconjugation stabilisation. They devised the following isodesmic reactions in 1-butyne and 1-butene to assess the hyperconjugation effect.
When hyperconjugative interactions are removed, virtual states with energies 4.9 and 2.4 kcal/mol higher than 1-butyne and 1-butene are produced. When these virtual states are used, conjugative stabilisation for 1,3-butadiyne is 9.6 kcal/mol and for 1,3-butadiene is 8.5 kcal/mol.
Hydrogenation
Unsaturated hydrocarbon hydrogenation is an exothermic process. The stability of unsaturated hydrocarbons improves as a result of hyperconjugation and resonance, with resonance accounting for
the majority of the increase in stability. The heat of hydrogenation is lower in a compound with the same number of ‘pi-‘bonds and greater stability. The energy released when a molecule is produced from its atoms is known as heat of formation. The more stable compounds have a greater heat of formation for isomers.
Inorganic Substrates
The Haber–Bosch process, which consumes an estimated 1% of the world’s energy supply, hydrogenated nitrogen to produce ammonia on a massive scale.
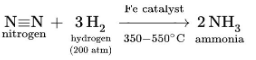
Although this technology has not been commercialised, oxygen can be partially hydrogenated to produce hydrogen peroxide. One issue is keeping the catalysts from causing the hydrogen peroxide to decompose into water.
Metal-Free Hydrogenation
Hydrogenation, for the most part, necessitates the use of a metal catalyst. Some hydrogen donors, such as diimide and aluminium isopropoxide, can be hydrogenated without the use of catalysts; the latter is demonstrated by the Meerwein–Ponndorf–Verley reduction. Academic study has looked into some metal-free catalytic systems. Tert-butanol and potassium tert-butoxide, as well as extremely high temperatures, are used in one such method for reducing ketones. The hydrogenation of benzophenone is represented in the diagram below:

This reaction is first-order in all three reactants, implying a cyclic 6-membered transition state, according to a chemical kinetics study.
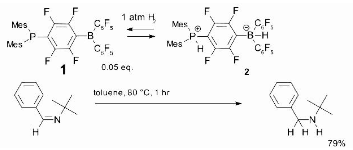
The phosphine-borane, compound 1, which has been dubbed a frustrated Lewis pair, is another mechanism for metal-free hydrogenation. At low temperatures, it reversibly takes dihydrogen to create
Fullerene, its mono-anion, ambient hydrogen, and UV light have been reported to catalyse the reduction of nitrobenzene to aniline.
Equipment Used For Hydrogenation
Batch Hydrogenation Under Atmospheric Conditions
The original and still widely used method of hydrogenation in teaching laboratories, this method involves introducing a solid catalyst to a round bottom flask of dissolved reactant that has been evacuated with nitrogen or argon gas and sealed with a penetrable rubber seal. A H₂ – filled balloon is then used to provide hydrogen gas. To facilitate mixing, the three-phase mixture is stirred. It is possible to track hydrogen uptake, which can be beneficial for tracking the development of a hydrogenation. This can be done using either a graduated tube filled with a coloured liquid (typically aqueous copper sulphate) or gauges for each reaction vessel.
Batch Hydrogenation At Elevated Temperature And/or Pressure
Because many hydrogenation processes, such as protecting group hydrogenolysis and aromatic system reduction, are extremely slow at ambient temperature and pressure, pressurised systems are preferred. In these circumstances, a catalyst is added to a reactant solution in a pressure vessel under an inert environment. The pressurised slurry is mechanically rocked to provide agitation, or a spinning basket is used. Hydrogen is added directly from a cylinder or built-in laboratory hydrogen source, and the pressurised slurry is mechanically rocked to provide agitation. High-pressure hydrogen generators have been developed as a result of recent improvements in electrolysis technology, which can manufacture hydrogen at pressures of up to 100 bar (1400 PSI) from water. Heat can also be employed since the pressure compensates for the reduced solubility of the gas.
Industrial Reactors
In a tubular plug-flow reactor (PFR) with a supported catalyst, catalytic hydrogenation is carried out. Although this varies depending on the catalyst, pressures and temperatures are often high. To increase activity, selectivity, and catalytic stability, various promoters are added to the metal, or mixed metals are utilised. Despite its low activity, nickel is widely used due to its low cost compared to precious metals.
Catalytic hydrogenation is also carried out in Gas Liquid Induction Reactors (Hydrogenators).
Acid Anhydride
An acid anhydride is a chemical substance formed when water molecules are removed from an acid. Organic acid anhydrides have the functional group R(CO)O(CO)R’ in organic chemistry. When one equivalent of water is removed from two equivalents of an organic acid in a dehydration reaction, organic acid anhydrides are formed.
An acid anhydride is an acidic oxide that interacts with water to generate an oxyacid (an inorganic acid that contains oxygen or carbonic acid) or with a base to form a salt in inorganic chemistry.
Conclusion
By destabilising the SOMO and stabilising the IE of the hydroxyethyl radical as compared to the hydroxym- ethyl radical, hyperconjugation explains the observed substantial alterations in the IE of the hydroxyethyl radical as compared to the hydroxym- ethyl radical.The hyper- conjugation energy increases by roughly 0.1 eV during geometry relaxation after ionisation, which explains the higher disparity between the vertical and adiabatic IEs.Hydroxyethyl is the opposite of hydroxymethyl. As a result, we can understand the significant difference in adiabatic and vertical IE in CH3CHOH represents the sum of the bonding interactions. Between the unpaired electron of carbon and the lone pair of oxygen And the interplay of hyperconjugation with CH Relaxed energy-Hyperconjugation is more efficient after ionisation, resulting in greater ionisation.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




