A hydration reaction is a chemical process in organic chemistry in which a hydroxyl group (OH-) and a hydrogen cation (an acidic proton) are added to the two carbon atoms bound together in the carbon-carbon double bond that forms an alkene functional group. The reaction is typically carried out in a highly acidic, aqueous solution. Hydration is distinct from hydrolysis in that hydrolysis divides the non-water component into two parts. The non-water component is not affected by hydration.
Hydration of Alkenes
The general chemical equation of the process for the hydration of alkenes is as follows:
RRC=CH2 in H2O/acid → RRC(OH)-CH3
A hydroxyl group (OH−) binds to one carbon of the double bond, and a proton (H+) binds to the other carbon. The process is highly exothermic. Following Markovnikov’s rule, the alkene functions as a nucleophile and attacks the proton in the first step. The second stage involves the formation of a link between an H2O molecule and the other, more strongly substituted carbon. At this moment, the oxygen atom has three bonds and is positively charged (i.e., the molecule is an oxonium). Another water molecule arrives and absorbs the additional proton.
This reaction produces several undesired byproducts (for example, diethyl ether in the process of producing ethanol) and is not considered very useful for the manufacture of alcohol in its simple form detailed here.
There are two approaches. To produce alkyl sulphate esters, the alkene is traditionally treated with sulfuric acid. This step can be stated as follows in the instance of ethanol production:
H2SO4 + C2H4 → C2H5-O-SO3H
This sulphate ester is then hydrolyzed to create sulphuric acid and yield ethanol:
C2H5-O-SO3H + H2O → H2SO4 + C2H5OH
The “indirect process” refers to this two-step procedure.
The acid protonates the alkene in the “direct procedure,” and water combines with the resulting incipient carbocation to produce alcohol. Because it is simpler, the direct technique is more popular. Phosphoric acid and a number of solid acids are among the acid catalysts. An example reaction pathway for the hydration of 1-methylcyclohexene to 1-methylcyclohexanol is shown below:
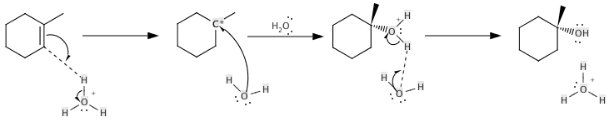
There are other additional routes for creating alcohols, including the hydroboration–oxidation process, the oxymercuration–reduction reaction, the Mukaiyama hydration, the reduction of ketones and aldehydes, and fermentation as a biological technique.
Ethanol Production by Hydration Of Ethene
Hydration is essentially the addition of steam to ethene in the presence of a phosphoric (V) acid catalyst, resulting in ethanol. High temperatures (300℃) and pressures (60-70atm) are the ambient requirements for hydration.
The chemical equation for ethene hydration, including the shown formula, is as follows:
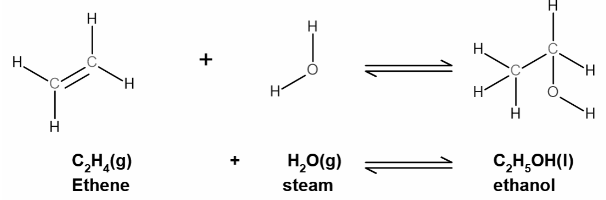
Even though the ethanol produced by hydration is theoretically pure, fractional distillation of the result is required to obtain pure ethanol. This is due to the presence of condensed steam within the collecting vessel.
Isopropyl Production
Isopropyl alcohol (IUPAC name propan-2-ol, also known as isopropanol or 2-propanol) is a colourless, flammable chemical compound with a strong odour (chemical formula CH3CHOHCH3). The simplest example of a secondary alcohol is an isopropyl group linked to a hydroxyl group, in which the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of ethyl methyl ether and 1-propanol.It is a common ingredient in antiseptics, disinfectants, hand sanitizers, and detergents, and is used in the production of a wide range of industrial and household chemicals. Globally, more than one million tonnes are produced each year.
Indirect hydration involves the reaction of propene with sulfuric acid to produce a mixture of sulphate esters. This process, which is common in the United States, can make use of low-quality propene. Because adding water or sulfuric acid to propene follows Markovnikov’s rule, these processes produce primarily isopropyl alcohol rather than 1-propanol. Following steam hydrolysis of these esters, distillation yields isopropyl alcohol. This process generates a significant amount of diisopropyl ether, which is recycled back into the process and hydrolyzed to produce the desired product.
CH3CH=CH2 + H2O ⟶ (CH3)2CHOH
Direct hydration is a high-pressure reaction of propene and water, either in gas or liquid form, in the presence of solid or supported acidic catalysts. This type of process usually necessitates higher-purity propylene (> 90%). In Europe, direct hydration is more common.
Isopropyl alcohol can be made by hydrogenating acetone; however, this method requires an extra step compared to the others because acetone is normally made from propene via the cumene process. The cost of IPA is primarily determined by the cost of the raw material (acetone or propylene). The formation of MIBK and other self-condensation products is a well-known issue. Raney nickel was an early industrial catalyst, and modern catalysts are frequently supported by bimetallic materials.
Conclusion
Hydration is an important process in many other applications, such as the production of Portland cement through the water-induced crosslinking of calcium oxides and silicates. Desiccants function through the process of hydration.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




