The Hunsdiecker Reaction is a chemical reaction in which carboxylic acid silver salts react with halogens to generate an unstable intermediate, which is subsequently thermally decarboxylated to yield alkyl halides as the final product. Heinz Hunsdiecker and his wife Clare Hunsdiecker improved the reaction, and it became the most extensively used method for generating organic halides as a result of their work. The Hunsdiecker Reaction yields cyclopropanes or cyclobutanes, which can be utilised to synthesise steroids and alkaloids. The Hunsdiecker–Borodin reaction and the Borodin reaction are two names for the same phenomenon. It’s also an excellent example of both decarboxylation and oxidation.
Mechanism Of Hunsdiecker Reaction
The Hunsdiecker reaction’s mechanism, which includes the creation of the reactive intermediate, relies heavily on radical organic intermediates.
Decarboxylation results in the creation of a diradical pair.
Recombination of reactants is used to make the desired product.
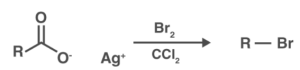
To break it down even more, the method starts with heating silver carboxylate in CCl4, which contains bromine. Due to the presence of bromine, the silver carboxylate transforms into acyl hypobromite during this reaction. After that, steady silver bromide precipitation occurs.
As a result, a radical chain reaction occurs, resulting in weaker oxygen-bromine bond homolysis. As a result of this reaction, the carboxyl radical and the bromine atom are generated. This carboxyl radical decarboxylates, creating a diradical pair of hydrocarbon or alkyl radicals, which then recombine to generate the required halide, in this case an alkyl bromide.
Sequence of Hunsdiecker Reaction
This is one of the first decarboxylative radical-intermediate producing reactions, and it has been shown to be useful in the arylation, acylation, and alkylation of certain compounds. It’s simple to set up, requires moderate conditions, employs low-cost starting materials, has a straightforward workup, provides good yields, and creates minimal byproducts. We must, however, maintain a level of familiarity with Hunsdiecker’s eccentricities.
It was also a driving force behind the development of closely related transformations like the Menisci response.
Factor Affecting Reaction Time
Some of the factors that influence reaction time are as follows:
1.Temperature-:
A 100° increase in temperature nearly doubles or triples the reaction rate in a homogeneous process in most cases. In certain cases, the increase in reaction rates is even characterised as being greater.
2.Number of reactant in the mixture-:
In the absence of a catalyst and at constant temperature, the indicated reaction rate increases as the concentration of reactants increases. The number of molecules per unit volume increases as the concentration of the reactant increases. As a result, collisions become more often, resulting in a faster reaction time.
3.Nature of Reactants
A chemical reaction arranges the atoms between the interacting molecules and the result. Here, old bonds are shattered and new bonds are formed. As a result, the strength and type of the bonds in the reactant molecules have a big influence on how quickly they turn into products. The reaction with a smaller quantity of bond rearrangement takes less time than the reaction with a larger amount of bond rearrangement.
4.Catalyst
The pace of the chemical reaction increases in the presence of a catalyst, resulting in a quicker chemical reaction.
5.Reaction
Light Reaction The rate of chemical reactions speeds up when reacting molecules absorb specific wavelengths of radiation, and these reactions are known as photochemical reactions.
Importance of Hunsdiecker Reaction
It was once used to make a carbocyclic ring that could not be made using only a Grignard reaction. It is capable of producing cyclopropane rings from alcohols and alkynes, which is extremely difficult to achieve using other methods.
In some cases, it can produce two different types of alkyl halides from the single benzene molecule. Chemists can employ this process to create complex organic compounds with a low cost and high atom economy.
A metal-halogen exchange is required in many organic processes, including the Suzuki Coupling, Kumada Coupling, and Heck Reaction. The Hunsdiecker Reaction yields cyclopropanes or cyclobutanes, which can be utilised to synthesise steroids and alkaloids
Conclusion
We conclude following points from the above topic -:
1.In the Hunsdiecker Reaction, carboxylic acid silver salts react with halogens to generate an unstable intermediate, which is then thermally decarboxylated to yield alkyl halides as the final product.
2.When Alexander Borodin used silver acetate to produce methyl bromide in 1861, he was the first to demonstrate this reaction type.
3.The concentration of the components involved influences the rate of a reaction.
4.The Hunsdiecker reaction’s mechanism, which includes the creation of the reactive intermediate, relies heavily on radical organic intermediates.
5.Light The rate of chemical reactions speeds up when reacting molecules absorb specific wavelengths of radiation, and these reactions are known as photochemical reactions.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




