Certain conductivity, also known as conductivity of an electrolytic solution at a specific concentration, is the conductance produced by a unit volume of solution held between two platinum electrodes separated by a unit length and with a unit cross-section. The conductivity of a solution diminishes with dilution because the number of ions per unit volume that carry the current in a solution decreases. The ability of a solution to conduct current is known as conductivity. The Greek letter kappa is used to signify it (k).
Variables that influence Conductivity
It’s a very sensitive physical quantity that’s influenced by a wide range of variables. The following are some of these factors.
- An electrolyte’s chemical composition
- For ions, the molecular mass is measured in millimetres
- The strength of a solution’s concentration in terms of concentration
- Temperature
- The solvent’s chemical composition
Molar Conductivity
A substance’s molar conductivity is a measurement of how well it conducts electrical current or water.
The conductance of volume V of a solution containing one mole of electrolyte at a certain concentration is defined as the conductance of volume V of a solution containing one mole of electrolyte held between two electrodes with an area of cross-section A and a distance of unit length.
Molar conductivity = ⋀m = k/cWhere,
⋀m = molar conductivity
c = Specific conductivity
C = concentration in moles per volume
Molar conductivity is calculated using the equation below:
⋀m (S cm2 mol-1) = k(S/cm)×1000/molarity(mol/L)
Variation of Conductivity
Electrolytes are divided into two categories: strong and weak. Strong electrolytes typically undergo complete ionisation, resulting in better conductivity than weak electrolytes that only experience partial ionisation. The molar conductivity of strong electrolytes, such as salts, strong acids, and strong bases, is relatively moderately affected by concentration. The molar conductivity of a strong electrolyte increases with dilution due to a decrease in solute–solute interaction. Friedrich Kohlrausch (around 1900) suggested the non-linear law for strong electrolytes based on experimental data:
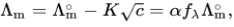
Where,
Λ∘m is the molar conductivity at infinite dilution
K is the Kohlrausch coefficient,
α is the dissociation degree
fλ is the lambda factor
Molar Conductivity
Molar Conductivity is defined as the product of the Conductivity of a solution of an electrolyte divided by the Molar concentration of the electrolyte, and it is used to determine the efficiency with which an electrolyte conducts electricity in solution when it is assigned to a specific electrolyte. To put it another way, Molar Conductivity is the total conducting power of all the ions that are formed when a mole of electrolyte is dissolved in a solution.
Specific Conductance
The specific conductance (L) of an electrolytic solution is the conductance of the solution contained between two electrodes that are one centimetre apart and have a one centimetre square surface area. In other words, the conductance of a 1 cc solution enclosed between two electrodes that are one centimetre apart can be defined as the L. The unit of L is equal to ohm-1 cm-1 or siemens m-1.
The specific conductance (L) of a solution is determined by the amount of ions present per cubic centimetre of solution, the charge of the ions, and the speed at which the ions move. It is also dependent on the temperature of the electrolytic solution, with each degree Celsius increase in temperature resulting in an increase of 2 percent. As a result, when the strong electrolyte solution is diluted, the quantity of ions per cc reduces but the speed of the ions increases, as seen in the graph. However, the first element takes precedence over the second, and L decreases when the solution is diluted.
Conclusion
The presence of free ions in electrolytes leads the electrolytes to conduct electricity due to their proclivity to conduct electricity. It’s similar to how free electrons improve the conductivity of electricity in metallic conductors, which happens in both circumstances. When it comes to electrical current, the Arrhenius equation or principle is utilised to define electrolytic conduction.
Electrolytic solutions, which are generated by dissolving a variety of salts in a solution and allowing the solution to evaporate, are well-known. In order to work correctly, the salts do not have to be ionic all of the time. The only stipulation is that the compound be made up of ions that have diametrically opposed charges.
When an electrolyte is dissolved in water, the electrolyte molecules are split into two unique charged ions, each with a different charge, according to the Arrhenius principle.
The charged particles in the solution are free to move about in their surroundings. In order to lessen the charge on their own surface, positive ions, also known as cations, can migrate towards a negative electrode, also known as a cathode. Positive ions will attract toward the positive electrode or anode during the oxidation process while also oxidising themselves. Electric conduction is caused by the passage of charged particles across a fluid medium.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




