Sugar having six carbon atoms and one aldehyde group, glucose is a simple sugar with no further chemical constituents. Monosaccharide with the chemical formula C6H12O6 is what we’re talking about.
It is also referred to as dextrose. It is referred to as aldohexose due to the fact that it has 6 carbon atoms and an aldehyde group in its structure. It can be opened in two different ways: as an open-chain structure or as a ring structure. Animals’ livers and kidneys are responsible for its production. It can be found in plants in a variety of forms, including fruits and other portions of the plant. D-glucose is the type of glucose that occurs naturally in the body. In either solid or liquid form, it is possible to come upon this substance. Acetic acid is a soluble in water compound that can also be dissolved in water. It has no odour and has a nice taste to it. Glucose was first extracted from raisins in the year 1747 by Andreas Marggraf, a German scientist. Jean Baptiste Dumas was the first to use the term glucose, which was coined in 1838.
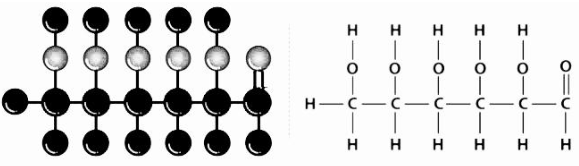
Structure
Properties of Glucose
C6H12O6 | Glucose |
Molecular Weight/ Molar Mass | 180.16 g/mol |
Density | 1.54 g/cm³ |
Melting Point | 146 °C |
Simple sugar | Monosaccharide |
Sugar can be referred to either aldohexose or dextrose, depending on the context. It is a monomer of many bigger compounds, such as carbohydrates, starch, and cellulose, and it has a crystalline structure. This is the organic substance that is found in the greatest quantity on the planet. It was determined that the structure depicted above was appropriate based on the evidence presented below:
- Its molecular formula is C6H12O6, which stands for carbon, hydrogen, and oxygen.
- When HI is heated over an extended period of time, the compound n-hexane is created, indicating that all six carbon atoms are bonded together in a straight chain.
- The oxime is created when glucose combines with hydroxylamine and cyanohydrins when hydrogen cyanide is added to it, resulting in the formation of oxime. The existence of the carbonyl group in glucose can be confirmed using this reaction.
- In the presence of a mild oxidising agent such as bromine water, glucose is oxidised to carboxylic acid, which has six carbon atoms, and this is known as carboxylation. In this case, the carbonyl group is present in the form of a carboxylic acid group.
- The existence of the -OH group is confirmed by the acetylation of glucose with acetic acid, which results in the formation of glucose pentanoacetate.
- When glucose and gluconic acid are both oxidised with nitric acid, dicarboxylic acid and saccharic acid are produced. This indicates the existence of primary alcohol in the system.
Preparation Of Glucose
The two main sources of glucose are sucrose (cane sugar) and starch.
- Sucrose or cane sugar preparation:
Sucrose, often known as sucrose, is a disaccharide having the formula C12H22O11. Glucose and Fructose are generated in equimolar amounts in a boiling aqueous solution of sucrose with dilute HCl or dilute H2SO4.
C12H22O11 + H2O → C6H12O6 + C6H12O6
Sucrose Glucose Fructose
- Starch-based preparation:
It’s a polysaccharide that produces glucose when cooked with dilute H2SO4 at 393 K under 2 to 3 atmosphere pressure.
( C6H12O5)n + n H2O → nC6H12O6
Starch Glucose
Uses Of Glucose
- It is used to treat hypoglycemia (low blood sugar) (low blood sugar)
- It is given to people who are really ill and unable to eat since it contains carbohydrates and calories.
- It is employed in the treatment of elevated potassium levels in the bloodstream (hyperkalemia)
- It is utilised as a precursor in the synthesis of a variety of compounds.
Conclusion
Glucose is the monosaccharide with the greatest abundance. Glucose is also the aldohexose that is most commonly found in the bodies of most living species. The fact that glucose has a lower potential than other aldohexoses to react nonspecifically with the amine groups of proteins could be one explanation for this. Glycation is a chemical reaction that weakens or destroys the activity of numerous proteins, as shown, for example, in glycated haemoglobin.
According to the National Institutes of Health, glucose has a lower rate of glycation than other aldohexoses because it has a more stable cyclic form, which means it spends less time in its reactive open-chain form than they do. One of the reasons for glucose’s ability to exist in the most stable cyclic form of all the aldohexoses is that all of its hydroxyl groups (except from the hydroxyl group on the anomeric carbon of d-glucose) are located in the equatorial position (see Figure 1). The fact that glucose is the most abundant natural monosaccharide is probably due to the fact that it is less glycated with proteins than other monosaccharides. Another possibility is that glucose, as the only d-aldohexose that has all five hydroxy substituents in the equatorial position in the form of -d-glucose, is more readily available to chemical processes because it contains all five hydroxy substituents in the equatorial position.
Glucose, often known as blood sugar, is the primary sugar found in your blood. It is derived from the foods you consume and serves as your body’s primary source of energy. Your blood transports glucose to all of the cells in your body, where it is converted to energy. Diabetes is a condition in which your blood glucose levels are abnormally high.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




