In organic chemistry, a functional group is a collection of atoms or bonds inside a substance that is responsible for the substance’s specific chemical reactions. A functional group is made up of atoms or bonds that are responsible for the substance’s unique chemical reactions. The same functional group will behave and react in the same way regardless of the chemical it is located in.
Two molecules with the same functional groups but different sizes might participate in chemical processes that are comparable or identical. The presence of a functional group in a molecule implies that its behaviour and chemical reactions may be predicted with great precision. Proteins, carbs, and lipids all contain functional groups.
By analysing the properties of various functional groups and their interactions, it is possible to design the chemical synthesis process, which entails the planned execution of chemical processes in order to manufacture a certain product.
Role of Functional Groups
Organic Chemistry Functional Groups
By utilising the services of additional functional groups, the manner in which the functional groups participate in a chemical process can be further adjusted, and these functional groups can also be interconverted.
A few functional groups using carbon are depicted in the following diagram:
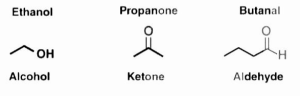
As a result, it is possible to understand that functional groups are moieties that have their own specific traits and properties that are independent of the molecule to which they are bound.
- Covalent bonding is a chemical bond that connects the atoms of these groups, as well as the group as a whole, to the molecule.
- For polymers, the functional groups are typically bonded to the nonpolar cores of the carbon atoms in each repeating unit of the corresponding polymer, imbuing the carbon chain with certain chemical properties.
- It has been noted that some functional groups have an ionic charge attached to them, as in carboxylate salts that contain the -COO– ionic group.
- When these groups are linked to molecules, they transform the molecule into either complexions or polyatomic ions, depending on their composition.
- The functional group that is bound to the central atom is referred to as a ligand in a coordination complex.
Some more functional groups comprising components such as nitrogen and oxygen, as well as alternative hybridizations of the carbon-nitrogen and carbon-oxygen bonds, are illustrated in the next section:
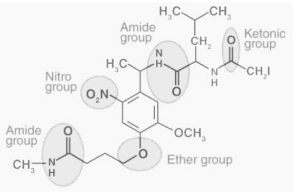
The existence of functional groups in a molecule has an effect on the solubility of the molecule in question as well as its potential to form complexes with other molecules. The solubility of a solution rises when the functional groups of the solute and the solvent interact well. For example, because the -OH (hydroxyl) group is present in both sugar and water, sugar can be easily dissolved in water when the two are combined.
The presence of a highly electronegative functional group connected to a less electronegative atom or molecule results in the formation of polarity, which allows the initially nonpolar molecule to be soluble in water or other aquatic environments.
Conclusion
In organic chemistry, a functional group is a cluster of atoms within a molecule that bond together to respond in predictable ways. Functional groups include the groups hydroxyl, ketone, amine, and ether, to name a few examples.
When one or more atoms are joined together, they form functional groups, which have specific chemical properties that are independent of what is linked to them. Covalent bonds connect the atoms of functional groups to one another and to the rest of the molecule, allowing them to act as a unit. Ligands are functional groups in a coordination complex that interact with a central atom and form a bond with it.
In the case of carbon atoms connected to functional groups, the first carbon atom is referred to as alpha carbon; the second carbon atom is referred to as beta carbon; and so on. The same way, a functional group can be classified as primary, secondary, or tertiary according to how many carbon atoms it is related to (one, two, or three).
Functional groups in organic chemistry are specific groups of atoms within molecules that are responsible for the chemical reactions that are characteristic of those molecules. In organic chemistry, functional groups are responsible for the chemical reactions that are characteristic of those molecules. No matter how large or little the molecule is, the same functional group will experience the same or nearly same chemical reaction regardless of its size (s).
Organic molecules’ functional groups are made up of groups of atoms that are bonded to the carbon backbone of the molecule. The chemical reactions that distinguish organic substances are responsible for the formation of functional groupings. They are less stable than the carbon backbone and are more prone to participate in chemical processes as a result.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




