Fluorine is the twenty-fourth most abundant element in the universe and the thirteenth most abundant element on Earth. As a result of its use in smelting metal ores to lower their melting points, the mineral was given its name from the Latin word fluo, which means “to flow.” Fluorite is the primary mineral source of fluorine and the mineral that gave the element its name was first described in 1529. Fluorine was first proposed as an element in 1810, but it proved difficult and dangerous to separate from its compounds, resulting in the deaths or injuries of several early experimenters who attempted to do so. Only in 1886 did the French chemist Henri Moissan successfully isolate elemental fluorine through low-temperature electrolysis, a process that is still used today for modern production of fluorine. During World War II, the Manhattan Project began commercial production of fluorine gas for use in uranium enrichment, which is the gas’s most important application.
Due to the high cost of refining pure fluorine, the vast majority of commercial applications rely on fluorine compounds, with steelmaking accounting for approximately half of all mined fluorite. The remainder of the fluorite is converted into corrosive hydrogen fluoride, which is then used to make various organic fluorides, or into cryolite, which is used in the refining of aluminum and other metals. A carbon–fluorine bond is frequently found in molecules, and this bond is responsible for their excellent chemical and thermal stability. Their primary applications include refrigerants, electrical insulation, and cookware, the latter of which is known as PTFE (Teflon). C-F bonds are found in a number of pharmaceuticals, including atorvastatin and fluoxetine. The fluoride ion produced by dissolved fluoride salts helps to prevent dental cavities, and as a result, it is used in toothpaste and municipal water fluoridation. Fluorochemical sales are worth more than US$69 billion per year all over the world.
Fluorocarbon gasses, in general, are greenhouse gasses with global warming potentials ranging from 100 to 23,500 times greater than that of carbon dioxide, and SF6 has the highest global warming potential of any known substance, with a global warming potential of 23,500 times greater than that of carbon dioxide. Because of the strength of the carbon–fluorine bond, organofluorine compounds are known to persist in the environment for long periods of time. Fluorine does not appear to play a role in the metabolism of mammals; however, a few plants and sea sponges produce organofluorine poisons (most commonly monofluoroacetate) that are used to deter predation.
Properties of fluorine:
- Fluorine is a naturally occurring element in the earth’s crust, and it can be found in coal, clay, and rocks.
- Hydrogen fluorides are released into the atmosphere by industries as a result of combustion processes.
- Fluorine is present in the atmosphere in the form of organic chloride compounds and salt spray at a concentration of 0.6 parts per billion (ppb).
- In urban environments, the element has been detected at levels of approximately 50 parts per billion (ppb).
Uses of fluorine:
- In semiconductor manufacturing, molecular fluorine and atomic fluorine are used for plasma etching, MEMs fabrication, and flat panel display production, among other applications.
- Chlorofluorocarbons are commonly found in air conditioners and refrigerators, among other places.
- Fluorides are also added to toothpaste in order to prevent dental cavities from forming in the mouth.
- The metal could be used to map the circulatory system and any abnormalities that may be found therein.
- It has been proposed that this material be used in optoelectric nuclear batteries.
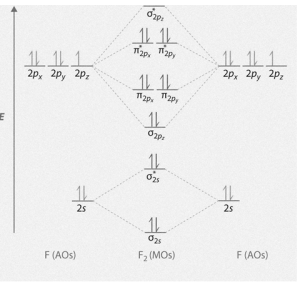
Orbital diagram of fluorine:
Conclusion:
Fluorine is a chemical element with the symbol F and atomic number 9. It is found in nature in small amounts.Fluorine is the twenty-fourth most abundant element in the universe and the thirteenth most abundant element on Earth.
Fluorocarbon gases, in general, are greenhouse gases with global warming potentials ranging from 100 to 23,500 times greater than that of carbon dioxide, and SF6 has the highest global warming potential of any known substance, with a global warming potential of 23,500 times greater than that of carbon dioxide.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






