Ferric chloride is a solid that ranges in colour from orange to brown-black. It is only marginally soluble in water. It is not flammable. It is corrosive to aluminium and most metals when wet. Before adding water, pick up and remove any spilled solids. It is used in the treatment of sewage, industrial waste, water purification, as an etching agent for engraving circuit boards, and in the production of other chemicals.
The solution of ferric chloride is a colourless to light brown aqueous solution with a faint hydrochloric acid odour. Most metals are highly corrosive, and tissue is likely to be corrosive as well. It is used in the treatment of sewage and the purification of water.
What is Ferric chloride-FeCl3?
Ferric chloride, also known as iron(III) chloride, is a vital chemical that is used in a variety of industries. The oxidation of ferrous chloride with chlorine results in the formation of iron(III) chloride as an aqueous solution. The primary application of ferric chloride is to remove impurities from water and to treat wastewater.
Total water treatment accounts for approximately 80% of total ferric chloride demand globally, including industrial water treatment applications and its use in pre-treatment of seawater prior to desalination. The production of printed circuit boards is the second-largest application, accounting for approximately 10% of total demand.
Other applications of ferric chloride include its use as a leaching agent in chloride hydrometallurgy, for example, in the production of silicon from FeSi (the Silgrain process), and as a catalyst in the reaction of ethylene with chlorine, which produces ethylene dichloride (1,2-dichloroethane), an important commodity chemical primarily used in the industrial production of vinyl chloride. Ferric chloride has the unusual distinction of being one of the purest and most concentrated forms of iron available on the market.
What is truly unique about ferric chloride is that it not only acts as a reactant to remove water impurities, but it also acts as a coagulant and flocculant. More popular information about ferric chloride can be found on the chemical of the month’s homepage.
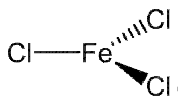
Structure of Ferric chloride
FeCl3 has the same structure as BiI3 in the solid state; each iron is octahedrally coordinated by six chlorines, as shown on the right.
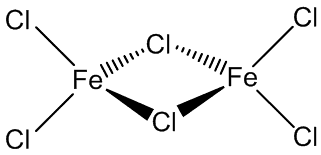
Above around 300ºC, it becomes volatile, and the vapour phase contains significant amounts of Fe2Cl6 molecules. The monomeric form of FeCl6 is present at higher temperatures, around 600ºC.
Ferric chloride test
The ferric chloride test is used to determine the presence or absence of phenol in a sample. It is a traditional phenol colorimetric test. It is carried out in the following manner –
- Neutralize a 1 percent ferric chloride solution with sodium hydroxide until a slight precipitate of FeO(OH) forms. It is filtered before being used.
- In a test tube, the organic substance or sample is dissolved in water, methanol, or ethanol.
- The above-mentioned neutralised ferric chloride solution is now added to the test containing the sample solution.
- If there is a transient or permanent purple, green, or blue coloration in the sample, it indicates the presence of a phenol or enol group.
Ferric Chloride Applications:
Ferric chloride is used in a variety of applications
- It is used in the manufacture of printed circuit boards.
- It serves as a catalyst in a variety of reactions.
- It’s commonly used in laboratories.
- It is used in phenol colorimetric tests.
- It can also test for gamma-hydroxybutyric acid and gamma-butyrolactone.
- In many reactions, it is used as a drying reagent.
- It is used in pattern welding by bladesmiths and artisans.
- It’s used to remove the aluminium coating from mirrors.
- It is employed in the etching of intricate medical devices.
Ferric chloride is primarily used to remove impurities from water, and it is also used in wastewater treatment. Ferric chloride is also one of the few existing water treatment chemicals that allows for the presence of odours. The most common iron salt used for coagulation is ferric chloride (i.e., FeCl3). Alum and ferric chloride can both be used to make various inorganic polymeric coagulants.
Uses of Ferric chloride
In the laboratory, anhydrous FeCl3 can be used instead of anhydrous AlCl3 in Friedel-Crafts reactions such as aromatic ring alkylation or acylation. Because it is a small, highly charged metal, it polarises reagents such as halogenoalkanes (alkyl halides), producing carbocations (carbenium ions) that attack benzene rings, as in this synthesis.
Conclusion
Ferric chloride is a solid that ranges in colour from orange to brown-black. It is used in the treatment of sewage, industrial waste, water purification, as an etching agent for engraving circuit boards, and in the production of other chemicals. Ferric chloride, also known as iron chloride, is a vital chemical that is used in a variety of industries. The primary application of ferric chloride is to remove impurities from water and to treat wastewater. Other applications of ferric chloride include its use as a leaching agent in chloride hydrometallurgy, for example, in the production of silicon from FeSi , and as a catalyst in the reaction of ethylene with chlorine, which produces ethylene dichloride , an important commodity chemical primarily used in the industrial production of vinyl chloride.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




