The existence of a polar molecule is also required for the dipole–induced-dipole forces. The second participating molecule does not have to be polar; nevertheless, if it is, this interaction enhances the previously reported dipole–dipole interaction. The presence of the partial charges of the polar molecule produces polarization, or In the dipole–induced–dipole interaction, the electron distribution of the other molecule is distorted. The second molecule obtains partial positive and negative charge areas as a result of this distortion, and so becomes polar.
The resulting partial charges behave like those of a permanently polar molecule and interact well with their counterparts in the polar molecule that generated them in the first place. As a result, the two molecules are able to cohere. This interaction also aids in the formation of intermolecular forces that cause hydrogen chloride gas to condense.
Definition of Dipole–Induced Dipole Forces
A dipole-induced dipole attraction is a weak attraction that occurs when a polar molecule causes a dipole to form in an atom or nonpolar molecule by disrupting the electron configuration in the nonpolar species.
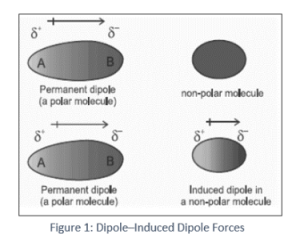
Below is a definition of terms used in the Dipole–Induced Dipole Forces
The key term are given below:
Polar molecules
A polar molecule is one with a charge on one side that is not cancelled out. There is a partial charge region on it. The positive side of the spectrum is slightly positive, while the negative side is slightly negative. They’re frequently lopsided, with an unequal distribution of electrons.
Permanent dipole
When two atoms bound together in a covalent connection have a considerable difference in electronegativity, permanent dipoles occur. As a result, the electrons in the shared pair are distributed unequally. They are attracted to the atom that is more electronegative.
Polarisability
When matter is exposed to an electric field, it has a tendency to acquire an electric dipole moment that is proportional to the applied field. Because matter is made up of constituent particles with an electric charge, such as protons and electrons, it is a property of all matter. When exposed to an electric field, negatively charged electrons and positively charged atomic nuclei are subjected to opposing forces and undergo charge separation.
Examples of Dipole–Induced Dipole Forces
Example: 1 an atom of Xenon a Xe atom’s electron dispersion around its nucleus is exactly spherical. However, because electrons are continually moving, it’s feasible for all of the electrons in some Xe atoms to be positioned on one side of the nucleus at any given time, resulting in a partially positive charge on one end and a partially negative charge on the other. As a result, these Xe atoms create an immediate dipole. The instantaneous dipole is likely to create a dipole in a neighboring atom during its transitory life, and the two instantaneous dipoles will attract each other.
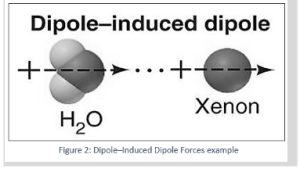
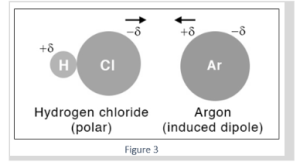
Example 2: When an argon atom approaches a polar HCl molecule, the electrons can move to one side of the nucleus, resulting in a very modest dipole moment that lasts barely a fraction of a second.
The reaction of hydrogen chloride (HCl) with argon (Ar). The positively-charged hydrogen (H) atom of one HCl molecule will attract electrons from the Ar atom, whereas the negatively-charged Cl atom of another molecule will attract the Ar nucleus in this type of interaction. As a result, Ar will develop a dipole moment.
Example 3: Polar O-H bonding exists in water. The negative O atoms attract the positive H atoms in surrounding molecules, generating a hydrogen bond, a type of dipole-dipole interaction that is extremely potent. Because water has hydrogen bonds, it has dipole-induced dipole and London dispersion forces.
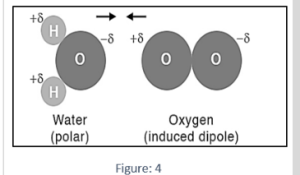
The attractive forces that keep extra oxygen in solution are analogous to the dipole-induced dipole forces previously outlined, but there are less water molecules available to produce dipoles in oxygen molecules. When water is supersaturated with oxygen, this causes the attraction of oxygen molecules to weaken.
Conclusion
In this article we learned that, between molecules with permanent dipoles, dipole–dipole forces exist (i.e., polar molecules). For molecules of identical size and mass, the intensity of these forces increases as polarity increases. When polar molecules form dipoles in nonpolar molecules, dipole–induced dipole forces are created. When the electrons of two neighboring atoms occupy places that cause them to create transient dipoles, the London dispersion force is a temporary attractive attraction. An induced dipole attraction is a term used to describe this force. The solubility of non-polar compounds in water is explained by the force of an induced dipole.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




