Introduction
Enzymes are made up of proteins which are the biocatalyst of our human body. Enzymes play a major role in increasing the rate of biochemical reactions by lowering down the activation energy. The first enzyme Zymase was discovered by Buchner and the term enzyme was coined by Kuhne. Northrop and Summer established the proteinaceous nature of the enzymes.Enzyme action
Enzymes possess active sites in which the reaction takes place. These have specific shapes. Enzymes remain unchanged up to the end of the reactions. It works by lowering the activation energy. An enzyme combines with the substrate to form an enzyme-substrate complex, which breaks up the enzyme and product. Enzyme molecules have a small portion that comes in direct contact with the substrate in the active site.Allosteric inhibition – Blocking of enzyme action by blocking its active sites
Allosteric enzyme
The term Allosteric means another site. Allosteric enzymes have an active site in them. There are several molecules in our body that affect enzyme regulation by increasing or decreasing enzyme activity. Enzyme regulation involves covalent and genetic modifications and allosteric regulations. Allosteric regulation controls cellular activities.Properties of allosteric enzymes
- These enzymes are complex and larger than the other enzymes. It contains subunits
- Allosteric sites are the binding sites of the enzymes, which differ from the substrate-binding sites and active sites
- Modulators are the molecules that bind to the sites
- The substrate-binding site which is present in the catalytic subunit is called the C subunit. The effector binding on the regulatory subunit is called the R subunit
Allosteric inhibition
Allosteric inhibition is the process in which the enzyme action gets blocked by the blockage of active sites. The other inhibitors join an enzyme at specific sites and change the form of the active site of the substrate. These sites are called the allosteric sites and the inhibitors are called the modifiers or modulators. The allosteric inhibition process is proposed by Jacob and Monod. Totally, there are two types of modulators such as- Positive modulators or activators
- Negative modulators or inhibitors
Mechanism of allosteric inhibition
Allosteric enzyme phosphor-fructokinase is activated by ADP and inhibited by ATP. Phosphate, Diphosphofructose, is activated by ATP and inhibited by AMP. Here, the form of the active site is altered, which results in the termination of the reaction. This phenomenon of inhibiting the reaction is called allosteric inhibition or allostery. The enzymes in the allosteric sites are called allosteric enzymes. Example : Glucose + ATP → Glucose 6- phosphate In the above reaction, hexokinase is an enzyme that converts glucose to glucose 6- phosphate. This reaction is called feedback allosteric inhibition.The catalytic action of enzymes
The reaction is simply the conversion of chemicals. The substrate is the chemical that is converted into a product. The tertiary structure of the proteins converts a substrate into the product. S → P But the substrate is already bound to the enzymes in the pocket or cleft. E + S → ES This process is called the transition phenomenon. The transition state structure is created when the substrate is bound to the enzyme active site. The bond is broken and the product is formed at the active site. The structure of the substrate will be altered into the product. Stability gives the energy status of the molecule. The formation of the ES complex is important for catalytic activity. This ES complex is short-lived and split down into the product and the EP complex. The EP complex is the unchanged enzyme intermediate formation. ES → EP → E + PSteps involved in the catalytic action
Step 1: The substrate binds with the active site of an enzyme. It fits into the site, forming the ES complexModel of enzyme action
Enzyme specificity
The position of the apoenzyme decides the specificity of the enzyme. The amino acid units are composed to form an enzyme apoenzyme. The tertiary structure of an enzymatic protein is folded several times to form a region called an active site. This active site has a correct molecular dimension and topology to compose and bind with a particular substance.Lock and Key hypothesis
On the basis of specificity, Fischer suggested the Lock and Key hypothesis for the enzyme action. The specific enzyme molecules are bound to a specific substrate molecule. According to this lock and key hypothesis, an enzyme has a particular shape called a lock. The particular lock shape is opened by a particular key, specially designed for it. This model gives the specificity of enzymes. Figure: Lock and key model
Figure: Lock and key modelInduced Fit hypothesis
The Induced Fit hypothesis was proposed by Koshland in 1959. According to Koshland, the active sites of an enzyme are not rigid. This hypothesis states that the combination of a substrate with an enzyme induces changes in the structure of the enzyme. Now, it is fit for the Enzyme-substrate interaction. The change in the shape of the enzyme breaks the bond, which promotes the reaction. Enzymes are enabled to perform their catalytic functions effectively.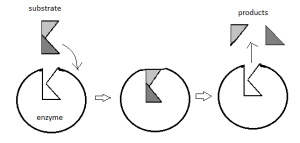 Figure: Induced fit hypothesis
Figure: Induced fit hypothesis
Enzymes commission number
An Enzyme’s commission number is called EC number, which gives a 4 digit code number to an enzyme. The four digits of Ec number are denoted as below,- First digit – Class
- Second digit – subclass
- Third digit – Sub Sub Class
- Fourth digit – enzyme number in sub sub class.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




