Introduction
The type of atoms or groups of atoms that leave the molecule are used to identify elimination reactions. Dehydrohalogenation is defined as the removal of a hydrogen atom and a halogen atom; dehalogenation is defined as the removal of both halogen atoms. Dehydration is the process of removing a water molecule from an alcohol; dehydrogenation is the process of removing both hydrogen atoms from the remaining atoms.Depending on the reaction kinetics, elimination reactions are categorized as E1 or E2. The reaction rate in an E1 reaction is proportional to the concentration of the substance to be transformed; in an E2, the reaction rate is proportional to both the substrate and the eliminating agent concentrations.
Types of elimination reaction
There are three types of elimination reaction
1)E1 type
2)E2 type
3)E1cb type
1)E1 type
- The reaction rate is related to the concentration of the molecule to be changed in a first-order kinetics
- two-step removal mechanism process, also known as unimolecular elimination
- creation of an intermediate
- regioselective and adheres to Zaitsev’s rule, which favours the creation of the Zaitsev product.
2)E2 type
a single-step removal mechanism
- sometimes referred to as a bimolecular reaction
- The carbon-leaving group and the carbon-hydrogen bonds both break off at the same time.
- Second-order kinetics states that the reaction rate is proportional to both the converted chemical and the transferring agent.
- stereoselective and regioselective are two types of selection.
3)E1cb type
- Unimolecular conjugate base elimination is a two-step elimination method.
- There is an acidic hydrogen atom in the compound, as well as a weak leaving group (e.g., -OH)
- In the dehydration reaction, there is a standard.
Elimination reaction of E1 and E2
E2 elimination reactions are carried out with relatively strong bases such alkoxides (deprotonated alcohols, –OR). Propene is formed when 2-bromopropane reacts with ethoxide, for example.
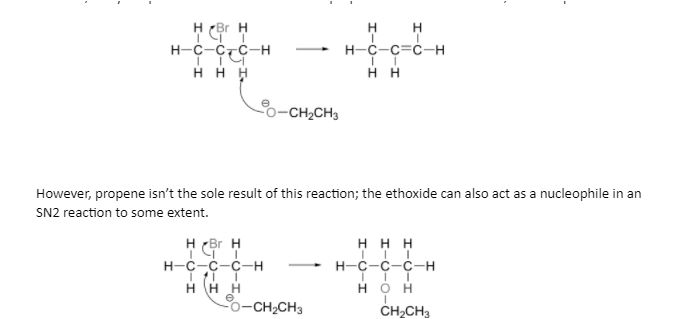
Because bases can also be nucleophiles and vice versa, chemists doing laboratory nucleophilic substitution or elimination processes must always be cognizant of the competition between the two mechanisms. A chemist, on the other hand, can sway the scales in one direction or the other by carefully selecting reagents. Because the electrophilic carbon is unimpeded and a favourable target for a nucleophile, primary carbon electrophiles like 1-bromopropane are considerably more likely to undergo substitution (by the SN2 process) than elimination (by the E2 mechanism).
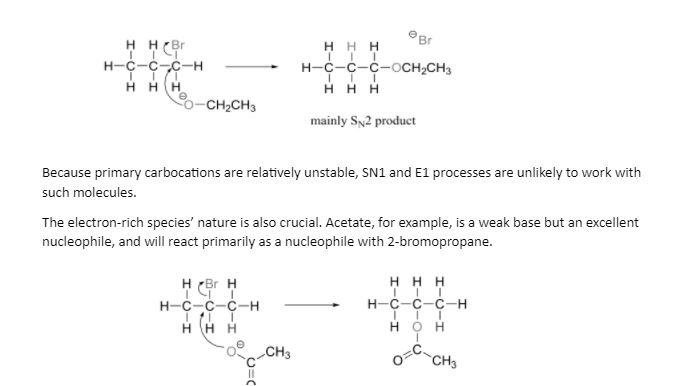
Heat is frequently employed to steer a reaction toward elimination rather than substitution. This is due to the fact that elimination increases the number of molecules (from two to three in the example above), and hence the entropy. This type of reaction, which has a considerable rise in entropy, is favoured by high temperatures. Because substitution rarely results in a considerable entropy change, if SN2 is required, the reaction should be carried out at the lowest temperature that allows substitution to proceed at a tolerable pace.
It’s also possible to utilise a strong hindered base like tert-butoxide. Because of the bulkiness of tert-butoxide, oxygen has a tough time reaching the carbon (in other words, to act as a nucleophile). It’s far more likely to grab a proton, which is much more accessible than the electrophilic carbon).

E1 reactions are characterized by the same carbocation-favoring circumstances as SN1 reactions: a secondary or tertiary substrate, a protic solvent, and a reasonably weak base/nucleophile. In fact, E1 and SN1 reactions usually happen at the same time, resulting in a combination of substitution and elimination products when a shared carbocation intermediate is formed. When tert-butyl chloride is mixed with ethanol and water, for example, it produces a mixture of SN1 (2-methylpropan-2-ol and tert-butyl ethyl ether) and E1 (2-methylpropene) products. If elimination is needed, heat is utilised, but
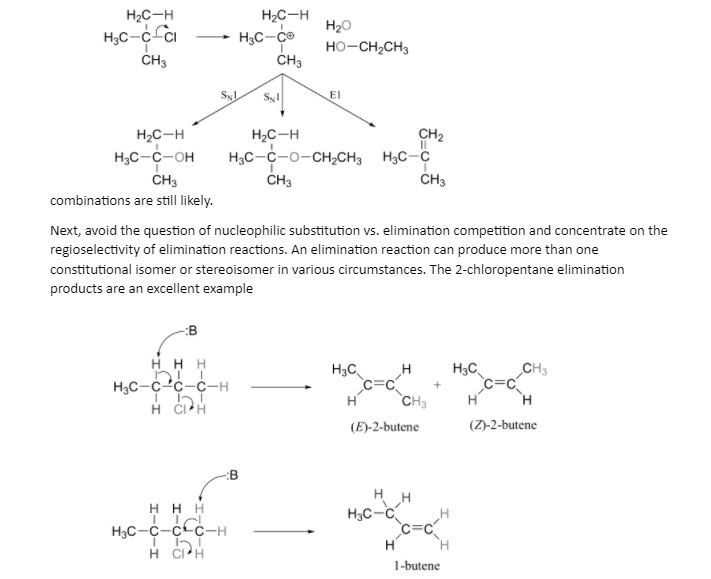
This is a regiospecific and stereospecific reaction. In general, more substituted alkenes are more stable, and as a result, the final mixture will contain less 1-butene than 2-butene (this is known as Zaitsev’s rule). Furthermore, because trans–alkenes are more stable than cis–alkenes, we can expect more of the trans product to form than the cis product.
However, due to steric considerations, certain additional eliminations (which we will not investigate) favour the least substituted alkene as the major result. The Hoffmann product is a type of product that is usually the polar opposite of the one predicted by Zaitsev’s Rule.
Conclusion
Any of a set of organic chemical reactions in which a pair of atoms or groups of atoms are removed from a molecule, usually by the action of acids, bases, or metals and, in some cases, by high temperature. Dehydrohalogenation is defined as the removal of a hydrogen atom and a halogen atom; dehalogenation is defined as the removal of both halogen atoms. The reaction rate in an E1 reaction is proportional to the concentration of the substance to be transformed; in an E2, the reaction rate is proportional to both the substrate and the eliminating agent concentrations. Types of elimination reaction There are three types of elimination reaction 1)E1 type 2)E2 type 3)E1cb type 1)E1 type The reaction rate is related to the concentration of the molecule to be changed in a first-order kinetics two-step removal mechanism process, also known as unimolecular elimination creation of an intermediate regioselective and adheres to Zaitsev’s rule, which favours the creation of the Zaitsev product.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



