In the substitution reaction, an electrophile substitutes for one of the hydrogens attached to the benzene ring. Benzene undergoes electrophilic substitution reactions that preserve aromaticity, rather than electrophilic addition reactions (the reactions characteristic of alkenes), which would destroy aromaticity. In electrophilic aromatic substitution of benzene, an electrophile reacts with an aromatic compound. In other words we can say that there is a substitution of an electrophile in place of a hydrogen of an aromatic compound.
The mechanism of electrophilic aromatic substitution of benzene involves two steps. The product of Step 1 (i.e., addition of the electrophile) is called an arenium ion intermediate or a Wheland intermediate. It is a carbocation intermediate consisting of five sp2-hybridised C atoms and one sp3-hybridised C atom.This intermediate then loses H+ through electrophilic elimination.
The Wheland intermediate is stable by resonance. However, it is still a lot less stable than the beginning material; this loss of aromaticity implies that the primary step is often the speed determinant step in EAS.
The second step regenerates the aromatic system, which is a quicker step. Many electrophiles (such as Br2) don’t seem to be sufficiently electrophilic to react independently. Such a large amount of EAS reactions require a catalyst to activate the electrophile. These catalysts are either Bronsted-Lowry acids or Lewis acids.
Electrophilic Aromatic Substitution Reaction
Numerous other functional groups can be added to the compound by this reaction. A purposeful cluster may be a substituent that brings bound chemical reactions that the hydrocarbon itself does not show.
So electrophilic aromatic substitution is a reaction in which an electrophile replaces an atom linked with an aromatic ring. So this is the substitute of a hydrogen atom with an electrophile linked with a benzene ring.
Mechanism
The mechanism mainly has two steps mentioned below.
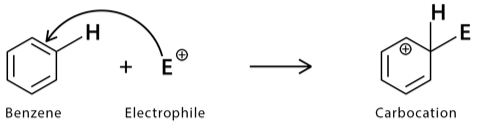
Step 1
The reaction starts with the electrophile attacking the pi-electron present in the aromatic benzene ring. It leads to the development of charged and delocalised cyclohexadienyl ions.
These ions are the arenium ions. This particle primarily has three resonance contributors. The electrophile attacks the aromatic ring.
This process is a very time taking and slow method. Due to the loss of aromaticity, there is a high energy activator there, and this reaction is endergonic.
Some of the critical factors that affect the electrophile reaction here are steric hindrance and chance.
Step 2
This step demands the removal of protons and fills with electrons of the arenium ion by a weak base.
A base attacks the positively charged carbon atom, leading to the loss of a proton. The electrons then want to reform a pi bond, and aromaticity is repaired.
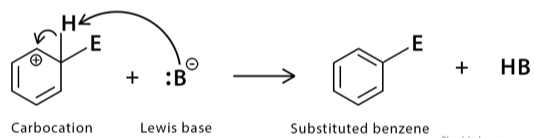
This reaction is a quick method and typically exergonic. Remember that the positively charged carbon atom loses a proton here as an electrophile is charging the benzene ring.
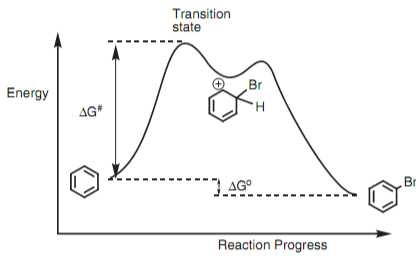
Energy Diagram of Electrophilic Aromatic Substitution Reaction
So here, we will discuss the reaction energy diagram of the reaction and how it looks.
The mechanisms consist of two steps, so the energy diagram has two energy barriers. Because the first step is rate-determined, its transition state is higher in energy. This intermediate participates in an electrophile elimination step in Step 2 of the mechanism. A proton is eliminated, and is assisted by the formation of a bond to a base that is present. Overall, then, E replaces H, converting benzene into a substituted benzene
Intermediates are determined and isolated in distinction. Transition states have a period of femtoseconds. While we can identify the transition states, we can never isolate them due to their short span.
The arenium ion intermediate is significantly less stable than either the reactants or products, due to the loss of aromaticity and the formation of a positively charged C that lacks an octet. We are aware that electron-donors increase the reaction speed (“activating”) while electron-acceptors decrease it (“deactivating”). Now, the replacement of isotopic chemical elements has minimal impact on the reaction rate. Hence, step 2 is not rate determining. As a result, Step 1 has a very-high-energy transition state and is the slow step.
The electron-donor and acceptor affect the nucleophilicity of the pi bond.
We can also introduce chlorine or bromine, reacting the element (Cl2, Br2) within the presence of the connected iron salt (FeCl3 or FeBr3) because of the Lewis acid catalyst.
However, since water from the air deactivates iron halides, it is common to use iron metal powder. The latter reacts simply with Cl2 or Br2 to make FeCl3 or FeBr3 severally.
The mechanism could be a typical electrophilic aromatic substitution, comprising electrophile activation. It could also be electrophilic addition to make the Wheland intermediate, and at last electrophilic elimination to lose H+.
Conclusion
So here, we have discussed the electrophilic aromatic substitution reaction, its mechanism, and its reaction energy diagram.Benzene and the electrophile both appear as reactants in the rate determining step, the rate of the overall reaction depends on the concentration of both species.The general electrophilic aromatic substitution reaction is first order with respect to the concentration of benzene and first order with respect to the concentration of the electrophile. It is the most vital reaction in the synthesis of organic chemistry. These reactions are useful to manufacture the precursors of some agricultural and industrial products.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




