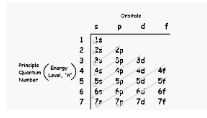
The s, p, d, and f orbitals are filled according to the element’s energy level and the number of valence electrons. The number of electrons in each of the four orbitals can vary. A single electron can fit in the s-orbital, while the other three orbitals can each hold up to six, ten, or fourteen electrons respectively. In general, the s-orbital denotes elements in groups 1 and 2, while the p-orbital denotes elements in groups 13, 14, 15, 16, 17, or 18, and the f-orbital denotes elements in groups lanthanides and actinides. The electron configuration of transition metals in the d-orbitals will be the primary focus of this module (d-block).
Transition metals’ electron configuration is unique in that it can exist in a variety of oxidation states. Regardless of how many different oxidation states an element can exhibit, the most stable elements tend to exhibit the same oxidation state. We will only focus on the first row of transition metals in this module; however, the other rows generally follow the same patterns as the first row.
Transition Elements of First Row:-
The following periodic table identifies the s, p, d, and f-orbitals:
Scandium (Sc), Titanium (Ti), Vanadium (V), Chromium (Cr), Manganese (Mn), Iron (Fe), Cobalt (Co), Nickel (Ni), Copper (Cu), and Zinc (Zn) are the 10 elements found in the first row of transition metals (Zn).
Scandium (Sc), Titanium (Ti), Vanadium (V), Chromium (Cr), Manganese (Mn), Iron (Fe), Cobalt (Co), Nickel (Ni), Copper (Cu), and Zinc (Zn) are the ten elements that can be found in the first row of transition metals: Titanium (Ti), Vanadium (V), Chromium (Cr), Manganese (Mn), Iron (Fe), Nickel (Ni).
Electronic Configuration Transition Elements of First Row:-
| Transition Element | Atomic Number | Electronic Configuration |
| Sc | 21 | [Ar]3d¹4s² |
| Ti | 22 | [Ar]3d²4s² |
| V | 23 | [Ar]3d³4s² |
| Cr | 24 | [Ar]3d54s1 |
| Mn | 25 | [Ar]3d54s² |
| Fe | 26 | [Ar]3d64s² |
| Co | 27 | [Ar]3d74s² |
| Ni | 28 | [Ar]3d84s² |
| Cu | 29 | [Ar]3d104s¹ |
| Zn | 30 | [Ar]3d104s² |
The following image shows the order for filling the subshells:
Order : 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p
Transition metals in room temperature :
Transition metals are excellent heat and electrical conductors. They are malleable and ductile, which means they can be formed into sheets and wires. Except for mercury (Hg), which is a liquid, they all have high melting and boiling temperatures and are solids at normal temperature.
Thermal conductivity of transition metals :
At low temperatures, transition metal carbides like TiC have very low thermal conductivities (K). Phonon scattering by conduction electrons significantly limits lattice conductivity at such temperatures. Point-defect scattering of phonons by carbon vacancies in non-stoichiometric materials dominates K at high temperatures. The electronic contribution is limited by the scattering of electrons by vacancies. Ambipolar diffusion increases the thermal conductivity of carbides over 10000C. At all temperatures, cemented carbides based on TiC (which is non-stoichiometric) have lower thermal conductivity than cemented carbides based on WC (which is stoichiometric). The thermal conductivity of cemented carbides is reduced as the binder phase percentage is increased.
High tensile strength and some other properties :
The tensile strength, density, and melting and boiling temperatures of transition elements are all high. This, like many other transition metal features, is related to the capacity of d orbital electrons to delocalize within the metal lattice. The more electrons shared between nuclei in metallic compounds, the stronger the metal.
Transition elements have a number of distinguishing characteristics:
They frequently produce colourful compounds.
They can exist in a range of oxidation states.
At least one of their compounds possesses a d-electron subshell that is incomplete.
They are frequently effective catalysts.
They are silvery-blue in colour when kept at room temperature (except copper and gold).
They are solids when kept at normal temperature (except mercury).
When they unite, they form complex ion complexes (aqua ions included).
They are usually diamagnetic in nature.
Atomic ionic radii of transition metals :
In the transition elements from group 3 to group 6, the atomic and ionic radii of the transition elements drop due to the inadequate shielding provided by the small amount of d-electrons in the transition elements. Those placed between groups 7 and 10 have atomic radii that are roughly similar, whereas those placed between groups 11 and 12 have atomic radii that are bigger. This is because the electron-electron repulsions cancel out the nuclear charge, resulting in a net neutral charge. In the course of progressing down the group, it is possible to see an increase in the atomic and ionic radii of the elements.
Ionisation enthalpy of transition metals :
The amount of energy that must be supplied to an element in order for a valence electron to be removed is referred to as the ionisation enthalpy. With an increase in effective nuclear charge acting on the electrons, an element’s ionisation potential increases proportionately to that increase in effective nuclear charge. As a result, the ionisation enthalpies of transition elements are typically higher than those of s-block elements. Transition elements are also more reactive than s-block elements. Interestingly, the ionisation energy of an element is inversely proportional to the atomic radius of the element. Atoms with lower radii have higher ionisation enthalpies than atoms with relatively larger radii, which is a general rule of thumb. As one moves down the row of transition metals, the ionisation energy of the transition metals increases (due to the increase in atomic number).
Conclusion :
The s, p, d, and f orbitals are filled according to the element’s energy level and the number of valence electrons. The number of electrons in each of the four orbitals can vary. A single electron can fit in the s-orbital, while the other three orbitals can each hold up to six, ten, or fourteen electrons respectively.The tensile strength, density, and melting and boiling temperatures of transition elements are all high. This, like many other transition metal features, is related to the capacity of d orbital electrons to delocalize within the metal lattice. The more electrons shared between nuclei in metallic compounds, the stronger the metal.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out