What are Electronic Configurations?
Electron Configurations is the symbolic representation of arrangements of the electrons of atoms in different atomic orbitals.
The arrangement of electrons determines how the atom will react with other atoms, particularly in what kinds of bonds it will form. A simple way to consider this is to view each electron as a tiny magnet and imagine what would happen if two magnets with opposite polarity were brought together.
Electron configurations are usually written with the lowest energy configuration at the top and higher energy levels (with more repulsion between the electrons) below. The superscripts are just numbers indicating which level that configuration is. So 1s2 indicates a single electron in the first (lowest) energy level. 2s2 indicates two electrons in the second level, one being in the same orbital as the other but with an opposite spin, so they are not repelling each other.
What do you mean by the electronic configuration of atoms?
In chemistry, an atom’s “electron configuration” is how its electrons are arranged in orbitals. It is usually written down as a series of quantum numbers, giving the number of electrons in a particular orbital. The quantum numbers are usually written as letters such as s, p, d, and f, standing for certain kinds of the orbital. The letter s stands for one kind of orbital: a single electron cloud surrounding the nucleus.
Electrons have a negative charge repel each other; they don’t like to be close together. But they can be forced into a small space by pulling the positively charged nucleus. When two or more electrons try to sit in the same small space, something interesting happens: their mutual repulsion changes into attraction.
We say that the electrons “bond” together to form a new kind of molecule with its own set of properties and behaviors.
Electron configurations are useful for describing the arrangement of electrons in atoms. Electron configurations are especially important when describing atoms in molecules.
The electronic configuration of an atom is the number of electrons it has arranged by energy level. A neutral atom has the same number of electrons as protons, but they don’t all have to be in the lowest energy level. The most common arrangement of an atom’s electrons is a closed shell or a filled subshell, but there are other arrangements that are also possible.
Rules for Electronic configuration
Afbau principle
It states that electrons of an atom in the ground state first fill the lowest energy subshells and then fill high energy subshells.
1s < 2s< 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p
Hund’s Rule
It states that an every orbital in a subshell is singly filled before any one orbital is doubly occupied and all electrons in singly occupied orbitals have same spin.
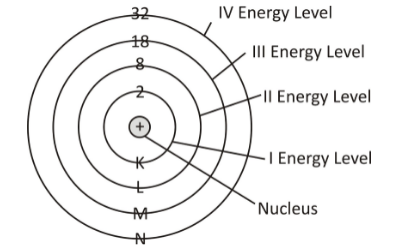
Subshell and shell
The subshells into which electrons are distributed are based on azimuthal quantum number .Each subshell is further divided into orbitals, each orbital holds two or more electrons, and each electron has a given spin quantum number. The set of orbitals held by a single electron is called a shell, and the electrons within a shell are said to be of the same principal quantum number. The elements with only one electron in their valence shell are very reactive as their outermost electron is very easily lost. On the opposite end of the scale lies noble gases, which possess 8 electrons.
Electronic Configuration of Elements:
Half filled and fully filled atomic orbitals are very stable as electrons are symmetrically distributed in them and exchange energy is maximum The exchange energy is the energy released during exchange process i.e If degenerate orbitals contain more than one electron with same spin then there is a chance for exchanging electrons positions. More exchanges means more energy is released. Let us take few example of elements having fully or half filled configuration and see how their electronic configuration can be written.
Electronic Configuration of Copper:
We are going to describe the electron configurations of some common atoms. Copper has 29 electrons, Copper has fully filled configuration .4s orbital has lowest energy and to be filled before 3d So, expected electronic configuration1s2 2s2 2p6 3s2 3p6 3d9 4s2
But to give coppers electronic configuration lowest energy possible,one electron from 4s jumps to 3d subshell which makes it completely filled and stable.
Electronic configuration of copper is [Ar] 3d10 4s1
Electronic Configuration of Chromium:
Chromium has atomic number 24. So, its expected electronic configuration is Ar] 3d44s2 But
the correct electronic configuration for chromium has been calculated as [Ar] 3d54s1. The 3d54s1 indicates a total of four electrons are present, and the two outermost orbitals (the 4d) are degenerate with two inner orbitals (the 4s), and therefore have a half-filled 4d subshell and a half-filled 4s subshell with an additional half-filled electron-pair that can be added to either orbital.
Conclusion:
So far in this study material, we have discussed what is the electronic configuration, electronic configuration of atoms, elements, copper, and chromium. The electronic configuration of an element is the organisation of electrons in orbitals around the atom’s nucleus.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




