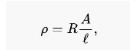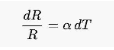Conductivity is a property of solids that may be tested to determine their electrical properties. When it comes to conductivity, it can be defined as the ease with which an electric current can pass through a particular substance. It is not true that all solids carry electricity in the same amount. Some of them have a high conductivity, whilst others do not conduct electricity at all, depending on their composition. Generally speaking, solids can be categorized into three categories based on their ability to conduct electricity:
Electric current can easily pass through conductors, which are solids that allow for the simple passage of electric current through them. Metals are often thought to be great electrical conductors.The presence of mobile electrons in metals is responsible for their electrical conductivity. The conductivity of metals is on the order of 107(m)-1, which is extremely high. Neither the conduction band nor the valence band are separated by any significant amount of space. Under the influence of an electric field, electrons can quickly transition from the valence band to the conduction band, resulting in them being excellent conductors of electricity.
Semiconductors:
In a semiconductor, there is a very small gap between the conduction band and the valence band; as a result, anytime sufficient energy is delivered to the electrons in a semiconductor, electrons will jump from the valence band to the conduction band. Because semiconductors conduct electricity more efficiently at higher temperatures, their conductivity varies between 10-6 and 10-4 (m)-1as the temperature rises. Semiconductors are classified into two categories: As a result of the application of heat to semiconductors, it is possible for them to expel electrons from their positions, leaving a positive hole in their place. Since the introduction of an electric field causes electrons to travel in one direction and holes to move in the opposite direction, semiconductors are now capable of carrying out electrical conductivity functions. Known as undoped or intrinsic semiconductors, these types of materials are used in a variety of applications. Silicon and germanium are two examples.
Extrinsic semiconductors are those that are not produced by the semiconductor industry.
A poor conductivity in their pure condition at ambient temperature is characteristic of silicon and germanium, according to the literature. In order to boost the conductivity of semiconductors, a little quantity of impurity is introduced into the semiconductor (group 13 and group 15 elements). Doping is the process of impregnating semiconductors with impurities in order to improve their conductivity. The semiconductors themselves are referred to as extrinsic semiconductors or doped semiconductors as a result of this process.
Insulators:
These materials do not conduct electricity and are therefore classified as insulators. The band gap between the valence band and the conduction band is extremely high in comparison to the valence band. It does not matter how much energy is applied to these solids; they do not have the ability to conduct electricity. For example, wood, plastics.
Dielectric Strength is the ability to conduct electricity.
It is a property of a substance that indicates the material’s capacity to endure high voltages when exposed to them.
The Temperature Coefficient of Resistance is a measure of how resistant something is to heat.
In a material, the temperature coefficient of resistance reveals how much the material’s resistance changes as a function of its temperature.
Thermoelectricity. When the junction, which is made by combining two metals, is heated, a minor voltage in the millivolt range is generated.
Resistivity
Electrical resistivity (also known as specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how well a material resists electric current. It is measured in ohms per meter of material thickness. A material with a low resistivity is one that readily allows electric current to flow through it. In most cases, the Greek letter is used to denote resistivity (rho). The ohm-meter (m) is the unit of electrical resistivity in the International System of Units. Consider the following example: A solid cube of material with sheet contacts on two opposite faces and a resistance between these contacts of 1 ohm corresponds to a resistivity of 1 m. The resistivity of the material is 1 m.
Ideal conditions exist when the cross-section of the studied material and its chemical and physical composition are homogeneous across its whole surface, and the electric field together with the current density are both parallel and constant throughout. The fact that a large number of resistors and conductors have a uniform cross section and a uniform flow of electric current, and that they are constructed of a single material, makes this an appropriate model for them. (See the illustration to the right.) As a result, the electrical resistivity rho (Greek: rho) can be computed by the following equation:
Where,
The electrical resistance of a uniform specimen of the material is denoted by R, and the length of the specimen is denoted by l
The specimen’s cross-sectional area is denoted by the letter A.
It is possible to express resistivity in terms of the SI unit ohm meter (m), which is equal to ohms multiplied by square meters (for the cross-sectional area) and then divided by meters (for the length).
Electric conductivity
Specifically, electrical conductivity, also known as specific conductance, is the inverse of electrical resistivity. The capacity of a material to conduct electric current is measured by its electrical conductivity. It is frequently represented by the Greek letter (sigma), but other letters such as (kappa) (which is particularly common in electrical engineering) and (gamma) are also sometimes used. Siemens per meter (S/m) is the unit of electrical conductivity used in the International System of Units (SI).
The coefficient of temperature
It is possible to characterize the relative change in a physical attribute that is linked with a particular change in temperature using the term “temperature coefficient.” The following equation defines the temperature coefficient in the case of a property R that changes when the temperature varies by dT:
In this case, has the dimension of an inverse temperature and can be written in several ways, for example, as 1/K or K1.
Conclusion
Charged particles (such electrons or protons) produce energy in the form of an accumulation of charge or a current.
Solids’ conductivity can be evaluated to discover their electrical properties. Conductivity is the ease with which an electric current can travel through a substance. Not all solids transport the same amount of electricity. Depending on their composition, some are very conductive while others are non-conductive.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out