Covalent bonds are the type of chemical bonds in which two electron pairs are shared by both atoms, and this is known as a double covalent bond.
In this type of covalent bond, four bonding electrons are formed between atoms rather than the usual two bonding electrons that are formed during the formation of a single bond. Because of the large number of electrons present in double covalent bonds, they are referred to as reactive bonds. Single bonds are longer and weaker than double bonds, which are much stronger.
When compared to single covalent bonds, double and triple covalent bonds are stronger. They are distinguished by the sharing of four or six electrons between the two atoms in question.
Double and triple bonds consist of sigma bonds between hybridised orbitals, and pi bonds among unhybridized p orbitals. Double and triple bonds provide additional stability to compounds by preventing any rotation around the bond axis of the compound.
Bond lengths between atoms that have multiple bonds are shorter than bond lengths between atoms that only have one bond.
Formation of a double covalent bond:
Double covalent bonds are formed when two atoms share four electrons in order to fulfil the octet rule, resulting in the formation of two covalent bonds. The octet rule states that atoms will share, lose, or gain electrons in order to achieve a total of eight valence electrons in a reaction. Usually, nonmetals such as carbon, nitrogen, and oxygen are used to form double covalent bonds with one another.
Electrons are free to move around an atom at high speeds and with little resistance. In accordance with their energy level and proximity to the nucleus, the electrons will maintain a specific shape in their orbits. Electron orbitals are the specific shapes that electrons take on as a result of their interactions. A double covalent bond is formed when s and p orbitals interact. There are four different types of electron orbitals, and they are all different shapes.
Some atoms donate more electrons to a double covalent bond than others, and some atoms donate far fewer electrons than others. They shuffle themselves around the electrons that are currently present in order to share four electrons.
Examples with simple molecules:
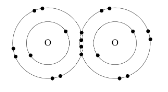
Oxygen, O2 : According to the illustration below, the two oxygen atoms can form a stable structure by sharing two pairs of electrons between themselves.
Ethane, C2H4 : Ethene is formed when two carbon atoms come together to form a double covalent bond.
Ethene is a compound composed of hydrogen atoms (1s1) and carbon atoms (1s² 2s² 2px¹ 2py¹).
Ethene is made up of four hydrogen atoms and two carbon atoms, and it exists as a single molecule before it is joined together.
It will now be possible to combine the different atomic orbitals that are pointing in the same direction to form molecular orbitals. Each orbit will contain a pair of electrons that are in a bonding relationship. Sigma bonds are the molecular orbitals that are formed by the end-to-end overlap of atomic orbitals in a molecular orbital molecule.
Conclusion:
Double bonds are formed when two atoms are joined together by a covalent bond. Double bonds are most frequently found between two carbon atoms, as in the case of alkenes, for example.Covalent bonds are the type of chemical bonds in which two electron pairs are shared by both atoms, and this is known as a double covalent bond.
In this type of covalent bond, four bonding electrons are formed between atoms rather than the usual two bonding electrons that are formed during the formation of a single bond.
Single bonds are longer and weaker than double bonds, which are much stronger.
Double and triple bonds consist of sigma bonds between hybridised orbitals, and pi bonds among unhybridized p orbitals. Double and triple bonds provide additional stability to compounds by preventing any rotation around the bond axis of the compound.
Ethene is made up of four hydrogen atoms and two carbon atoms, and it exists as a single molecule before it is joined together.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




