The London dispersion force is the weakest of the van der Waals forces, and it’s what causes nonpolar atoms or molecules to condense into liquids or solids as the temperature drops. Despite its weakness, the dispersion force is frequently the most prominent of the three van der Waals forces. Small, easily polarised molecules, such as water molecules, are an exception.
The role of light atoms, such as hydrogen, has been generally overlooked in discussions of molecule stability and reactivity. The physical and chemical properties of organometallic and inorganic compounds are influenced by these attractive forces, which are mainly between C–H moieties in auxiliary ligands. We begin with a review of many famous inorganic and organometallic complexes whose dispersion forces have been explicitly detected or calculated, and then move on to recent examples of organic species that have influenced contemporary thinking.
The following are significant words used by the London Forces:
Symmetrical
In chemistry, molecular symmetry refers to the symmetry found in molecules as well as the classification of these molecules based on their symmetry.
A symmetrical molecule is one whose appearance does not change when rotated around a symmetry axis; the original and rotated states are indistinguishable. An asymmetrical molecule, on the other hand, lacks a symmetry axis and can be rotated.
No dipole
Nonpolar molecules are those that have an even charge distribution but no dipole moment. Because CO2 is a linear molecule, our dipoles are symmetrical; their magnitudes are equal but their orientations are opposite.
Dispersion force
Dispersion forces are caused by the attraction between nearby molecules. The electron cloud of one molecule attracts the nucleus of another, causing the electron distribution to alter and a transient dipole to form.
Definition of Dispersion Forces or London Forces
“London dispersion dispersion forces, also known as dispersion forces, London forces, instantaneous dipole–induced dipole forces, Fluctuating dispersion forces.
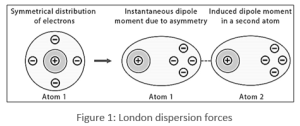
Types of bonds
Under the category of van der Waal forces, London dispersion forces include: They exist between all types of molecules, whether ionic or covalent, polar or nonpolar, and are the weakest of the intermolecular interactions. The more electrons a molecule contains, the stronger the London dispersion forces are.
Ionic bond
A chemical connection that forms when two ions with opposite charges come together. An ionic link is created when one atom gives up one or more electrons to another. These are the types of bonds present in salts, and they can form between atoms or between molecules.
Covalent bond
A coordinate covalent bond, also known as a dative bond, dipolar bond, or coordinate bond, is a type of covalent connection that has a specific shape is a two-centre, two-electron covalent bond in which both electrons originate from the same atom. The bonding of metal ions to ligands involves this type of interaction.
Examples of Dispersion Forces or London Forces
Nonpolar molecules exhibit London dispersion forces. These are some of them: Fluorine (F2), chlorine (Cl2), bromine (Br2), and iodine (I2), Helium (He), neon (Ne), argon (Ar), and krypton (Kr) are the Noble gases (Kr)
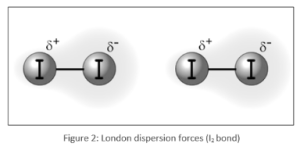
- For example: The only forces that exist between iodine (I2)molecules are the London dispersion forces, which are relatively weak. Because the strength of these forces is in line with iodine’s massive electron cloud and polarisability, it exists as a solid at ambient temperature.
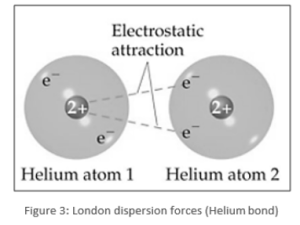
- For one helium atom, London dispersion forces cause a dipole to form on a neighbouring helium atom. Because it is a noble gas, helium will have the lowest boiling point because the only intermolecular forces present are dispersion forces, which are the smallest.
Formula Dispersion Forces or London Forces
The dispersion interaction, often known as the London interaction, is a formula that expresses the induced-dipole-induced-dipole interaction between molecules.
Polarisability refers to a molecule’s capacity to produce a charge separation or induced dipole.
The strength of the interaction between two dipoles can be described using the symbol μ. The electric field’s strength is precisely proportional to the strength of the force (E).
μ= α×E
represent induced dipole moment
represent polarisability
E represent Electric field
We may determine the energy of interaction using the London dispersion force formula.
V=32I4r2
Conclusion
In this article we learned, Intermolecular forces are significant because they influence a molecule’s physical properties such as melting point, boiling point, density, and fusion and vaporisation enthalpies. In both adhesion and sintering, London dispersion forces are relevant. The dispersion forces regulate the detailed geometry of the item at the crack tip and sintering neck during sintering.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



