Introduction
In Monosubstituted Benzene, the Functional Group Has A Direct Influence. When mono substituted benzene is electrophilically attacked, the rate of reaction and attack site differ depending on the functional group attached to it. Benzene, despite being an important industrial chemical, has limited application in consumer products due to its toxicity.
Benzene
One of the most basic petrochemicals is benzene, which is a natural component of crude oil. Benzene is classified as an aromatic hydrocarbon because of the cyclic continuous pi bonds between the carbon atoms. PhH is a common abbreviation. Benzene is a colourless, highly combustible liquid with a pleasant odour that contributes to the scent of gasoline (gasoline) stations. It is largely utilised as a precursor in the production of compounds with more complex structures, such as ethyl benzene and cumene, which are manufactured in billions of kilograms each year.
Benzene derivatives
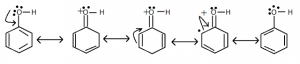
Why is NH2 more potent than OH in terms of activation?
Nucleophile, in other words, is a Lewis base. -OH or -NH2? Because Oxygen (O) has a higher electronegativity (the tendency of any element to attract a shared pair of electrons toward itself) than Nitrogen (N), N can easily donate its lone pair of electrons. As a result, NH2 has a higher nucleophilicity than OH.
In organic chemistry, what is the directed effect?
This direction is known as the directional or orientation effect, and it is determined by the type of the first substituent. The already existent substituent can raise or reduce the rate of subsequent substitution, i.e., it can activate or deactivate the benzene ring’s ability to undergo further substitution.
Reactions
The most typical benzene reactions involve the replacement of a proton by another group. The general method of derivatizing benzene is electrophilic aromatic substitution. Because benzene is sufficiently nucleophilic, it is substituted by acylium ions and alkyl carbocations, resulting in substituted derivatives.
1. Sulfonation, chlorination, nitration
Many functional groups are added to the benzene framework through electrophilic aromatic substitution. The usage of oleum, a combination of sulfuric acid and sulphur trioxide, is used to sulfonate
benzene. Detergents based on sulfonated benzene derivatives are beneficial.
When benzene combines with nitronium ions (NO2+), a powerful electrophile formed by mixing
sulfuric and nitric acids, nitronium ions (NO2+) are formed.
The precursor to aniline is nitrobenzene. In the presence of a Lewis acid catalyst, such as aluminium trichloride, chlorine is used to produce chlorobenzene.
2. Hydrogenation
Benzene and its derivatives are converted to cyclohexane and derivatives via hydrogenation. High hydrogen pressures are used in the presence of heterogeneous catalysts, such as finely split nickel, to induce this reaction. While alkenes and related chemicals can be hydrogenated at ambient temperature, benzene and related compounds require temperatures above 100 °C. This reaction is carried out on a huge scale in the industrial sector. Benzene is resistant to hydrogen in the absence of a catalyst. Because cyclohexene and cyclohexadienes are superior substrates, hydrogenation cannot be prevented. Birch reduction, a non-catalytic technique, hydrogenation benzene to the diene selectively.
Mechanism of Reaction:
- Di-substituted products are generated when mono substituted benzene is subjected to additional substitution. Ortho, para, and meta are the three types.
- The type of the substituents already present in the benzene ring determines which product is generated more frequently.
- This is referred as as substituent direct influence.
Ortho and para directing groups:
- They direct the incoming group to ortho and para positions. The rise in electron density at ortho and para positions is the cause.
- NH2, NHR, NHCOCH3, OCH3, CH3, C2H5 etc are some ring activating groups.
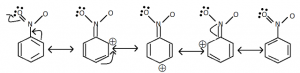
Meta-directing group:
- Because of the – I effect, halogens are a ring deactivating group.
- However, the electron density at 0 and P – positions is greater than at meta-position due to resonance.
- As a result, the directing group is O – and p –.
- That’s why these groups are known as deactivating groups.
Toxicity and Carcinogenesis:
Benzene and polynuclear hydrocarbons with more than two benzene rings fused together are poisonous and carcinogenic. Incomplete combustion of organic materials such as tobacco, coal, and petroleum produces these.
These are subjected to a variety of metabolic processes that damage DNA and result in cancer.
Conclusion
Ortho and para directing groups are electron releasing groups that guide the incoming group to ortho and para locations, where the electron density is higher. As a result, electrophilic substitution occurs primarily at these locations. At certain positions, the aromatic ring becomes reactive.
The electron density is lower in the meta state, hence it is less reactive. The electron withdrawing groups, on the other hand, are meta directing.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out