Intermolecular interactions are necessary for a molecule’s stability. There are various forms of intermolecular forces, and one of them is dipole-dipole forces. Dipole – Dipole Forces form of intermolecular force that occurs when a polar molecule interacts with a non-polar molecule. The approach of the polar molecule causes a charge separation in the electron cloud of the non-polar molecule in this interaction.
A polar molecule has an electric dipole moment due to the presence of partial charges on its atoms, as previously stated. Opposed partial charges attract one another, and if two polar molecules are oriented so that their opposite partial charges are closer together than their like charges, the two molecules will have a net attraction. At low temperatures, this form of intermolecular force helps hydrogen chloride to condense into a liquid. Because molecules rotate, they tend to remain in relative orientations with low energy, such as mutual orientation with opposite partial charges adjacent to one another, the dipole–dipole interaction leads to the weak contact between molecules in gases.
Definition of Dipole – Dipole Forces
The electrostatic forces between two permanent polar molecules are referred to as dipole-dipole forces or dipole-dipole interactions. In general, one molecule’s positive end attracts the negative end of another. The two molecules become closer as a result, increasing the substance’s stability. Because there is no transfer or exchange of electrons, this interaction differs from a traditional ionic or covalent bond.
There are several terminology used in the Dipole – Dipole Forces section, which is described below:
Delta (δ): A partial charge is represented by the lowercase Greek letter in molecular chemistry. This is always lower than the ion’s unit charge, but it has no true set value. A river delta gets its name from the shape of the upper-case letter delta.
Partial charges: The uneven distribution of electrons in chemical bonds produces partial charges. The shared electron oscillates between the connected atoms in a polar covalent bond, such as HCl. The partial charges that result are simply a property of zones within the distribution, not the entire assemblage.
Electronic charge: The action of elementary particles in response to an electric or magnetic field carries electric charge, which is a fundamental property of matter. The electric charge is composed of discrete natural units that cannot be created or destroyed.
Cause of Dipole-dipole interaction
The unequal distribution of electrons in a molecule causes dipole-dipole interaction. At one end of the molecule, the electrons gather. As a result, the molecule gains a partially negative charge on one end and a partially positive charge on the other, making it polar. Two polar molecules with opposite charges are attracted to each other by nature.

Examples of Dipole – Dipole Forces
Hydrogen chloride (HCl), hydrogen fluoride (HF), and water are all examples of dipole-dipole forces (H2O) which are given below:
- HCl (hydrogen chloride): HCl is a permanent dipole. The chlorine atom has a partially negative charge, while the hydrogen atom has a partially positive charge. When two HCl molecules are brought near enough together, the positive H of one attracts the negative Cl of the other, forming a bond.
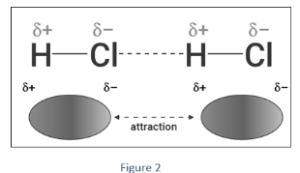
- Water (H2O): Two hydrogen (H) atoms are connected to an oxygen (O) atom in water (H2O). The O-H bond gains a permanent dipole as a result. The hydrogen atom has a partial positive charge, while the oxygen atom has a partial negative charge. As a result, the H from one molecule attracts the O from another, creating a dipole-dipole interaction.
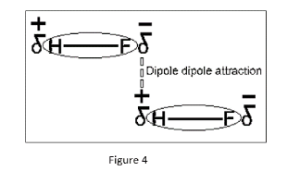
- Hydrogen fluoride (HF): (HF) and the dipole-dipole bonding that occurs when the poles of each molecule contact to form intermolecular bonds. The dipole-dipole bonding of HF. Take note of how the molecules’ opposite ends align to attract the little negative and positive charges.
Properties Dipole – Dipole Forces
- It happens when two permanent polar molecules come into contact with each other.
- The negative end of the spectrum attracts the positive end of the spectrum.
- 5 to 20 kJ per molecule bond strength
- Ionic and covalent bonding are weaker.
Conclusion
In this article we learned that, the attractive interactions between the positive ends of Dipole-dipole forces occur when one polar molecule interacts with the negative ends of another polar molecule.
The most essential intermolecular contact is dipole–dipole interaction, which aids medication solubilization in water. In covalent bonding, the dipole is caused by an uneven distribution of electron pairs. The electronegativity imbalance between linked atoms causes unequal sharing of electron pairs.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





