Dilution is the process of lowering the concentration of a solute in a solution, usually by adding more solvent to mix with it. When you add more solvent to a solution, like water, the concentration of a solute in the solution goes down. Diluting a solution means adding more solvent but not adding more solute to it.
If you want an exact amount of something, you can start with a high concentration solution and add water until you get the right concentration. Dilution is the name for this process. Mixing a high-concentration solution with a lower-concentration solution can also be used to dilute something. Because stock solutions are often bought and kept in very concentrated amounts, diluting solutions is a common task in the lab.
Dilution and Dilute Solution
Dilution is the process of diluting a solution by adding additional solvent. This method maintains the solute concentration constant while increasing the total volume of solution, hence decreasing the solute’s ultimate concentration. Additionally, dilution can be accomplished by mixing a solution with a greater concentration with a solution with a lower concentration. Diluting solutions is an essential procedure in the laboratory, as stock solutions are frequently acquired and stored in extremely concentrated forms. To be used in the laboratory (for example, in a titration), the solutions must be accurately diluted to a known, lower concentration.
The volume of solvent required to dilute a fresh solution to the desired concentration can be determined quantitatively. The following is the relationship
M1V1=M2V2
M1 signifies the original solution’s concentration, and V1 denotes the original solution’s volume; M2 denotes the diluted solution’s concentration, and V2 denotes the diluted solution’s final volume. It is critical to use the same units for volume and concentration on both sides of the equation when computing dilution factors.
Difference between solution and dilution
A solution is a homogenous mixture of two or more components with particles no larger than 1 nm in diameter. The concentration of a solution is defined as the amount of solute contained within it. In solutions, the solute and solvent concentrations are not equal. A solution can be, depending on the solute concentration,
- Dilute solution
- Concentrated solution
- Saturated solution
The terms dilute solution and concentrated solution are imprecise due to their lack of quantification. The primary distinction between dilute and concentrated solutions is that dilute solutions contain less solute while concentrated solutions contain more. Although these phrases do not convey quantitative information (real numbers), they are frequently beneficial when comparing solutions on a broader scale. Additionally, these phrases do not indicate whether the solution is saturated or unsaturated, nor do they indicate if the solution is “strong” or “weak.” These final two phrases will take on additional significance as we explore acids and bases.
| Dilute solution | Concentrated solution |
| When solute concentrations are low, a solution is described as “dilute.” | When there are a lot of solutes in a liquid, it is called a “concentrated solution.” |
| The salt that comes from a well is in a very small amount in the drinking water. | As more solute is added to a solution, the solution gets more concentrated. |
| more water can be added to a solution to make it less concentrated and easier to mix and spread out. | In a concentrated solution, there is a substantial quantity of water present. |
| There are a lot of solutes in a dilute solution, but only a small amount is in the solution at the same time. | A concentrated solution has a high concentration of the solute in it. |
Enthalpy of Solution
It’s called the enthalpy of solutions when two substances mix together. This is how much heat each substance takes or gives off when they mix. Endothermic reactions happen when there is a positive enthalpy in the solution. This means that heat is taken in and it feels cold to touch. Exothermic reactions happen when there is a negative enthalpy in a solution. This causes heat to be released and makes it feel hot to the touch. A lot of heat will be lost or gained if three separate processes don’t happen at the same time.
The first thing that needs to happen is for the solute, the substance that is being dissolved, to separate from the rest of the water. Perhaps you have some table salt, NaCl, in your pantry or kitchen cupboard. Sodium and chloride are attracted to each other, so NaCl is made up of sodium and chloride atoms that stick together. So, if you want to dissolve NaCl, you’ll need to use a certain amount of power. Enthalpy of this process is:
Two more things need to happen before the solvent can be used to dissolve something. Perhaps you want to mix your table salt with water. Water molecules are polar, which means that their partially negative poles stick to the partially positive poles of water molecules next to them because they are both polar. It takes a lot of work to separate them. Enthalpy of this process is:
This is the third thing that needs to happen. The solute and solvent need to mix together. Enthalpy of this process is:
The sum of these processes is equated as:
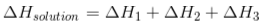
Conclusion
A solution whose concentration can be precisely measured is frequently required. Stock (or standard) solutions are defined as solutions with a known mass of solute in a known volume of solution. A volumetric flask, a piece of laboratory equipment, is required for the preparation of a standard solution. From 10 mL to 2,000 ml, these flasks are precisely calibrated to the same volume. Calibration marks can be found at the base of the stem. It’s important to know exactly how much solute is in the solution before adding it to the flask. The flask is then filled with solvent until the calibration mark is reached.
Often, it is more convenient to prepare a series of known concentrations by first producing a single stock solution, as indicated in the preceding paragraph. The stock solution can be diluted to any desired volume using aliquots (carefully measured volumes). In some circumstances, it may be difficult to correctly weigh a small amount of sample in order to prepare a small volume of a dilute solution. To get the necessary concentration in any of these cases, a solution must be diluted.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



