The diazonium salts (di denotes ‘two,’ azo denotes ‘nitrogen,’ and ium denotes that it is cationic in nature) or diazonium compounds are cationic salts (di denotes ‘two,’ azo denotes ‘nitrogen,’ and ium denotes that it is cationic in nature).
R is an alkyl or aryl group, and X is an organic or inorganic anion (for example, Cl–, Br–, BF4–, etc.).
As a result, they have two nitrogen atoms, one of which is charged. The diazonium salts include benzenediazonium chloride (C6H5N2+ Cl–), benzenediazonium hydrogen sulphate (C6H5N2+ HSO4–), and others.
Diazonium salts are one of the most flexible organic-inorganic combinations available. R-N2+ X– is a general way of representing it. The R stands for an organic group, usually an aryl group, and the X stands for ion.
Cl–, Br–, and BF4 are the most common X in diazonium salts. The presence of the N2+ group or the diazonium group gives these salts their names.
The suffix diazonium is added to the parent hydrocarbon from which these salts are produced, and then the anion X, such as bromide, is added after that.
The value of these chemicals can be seen in their applications and applications.
Preparation of Diazonium Salts
Diazotization or dissociation is the process of turning an organic chemical, usually primary aromatic amines, into diazonium salts.
The diazonium group has been shown to be highly unstable under normal settings, therefore it is rarely stored and is usually used immediately after production.
The reaction of nitrous acid with aromatic amines is one of the most common ways to make diazonium salt. Benzene diazonium chloride is formed when aniline (aromatic amine) reacts with nitrous acid to generate a diazonium salt.
Because nitrous acid is a highly poisonous gas, it is usually made in situ (during the procedure) by combining NaNO2 with a mineral acid.
Temperature is another significant factor that controls product formation during the preparation of these salts; most diazonium salts are stable below 5o C.
As a result, it’s critical to keep the reaction temperature below 5°C, or the diazonium group will disintegrate into N2 as soon as it’s created.
The reaction of aniline with NaNO2 and hydrochloric acid is shown below, giving benzene diazonium chloride as the end product.
The diazonium group has a wide range of applications. This mix of organic and inorganic components has been a benefit for scientists in a variety of fields, from dyes and pigments to the production of diverse organic molecules.
Chemical Reactions of Diazonium Salts
- Replacement by halide or cyanide ion:
The nucleophiles such as Cl–, Br–, CN– can be easily inserted in the benzene ring in the presence of Cu (I) ion. This reaction is often known as the Sandmeyer reaction.
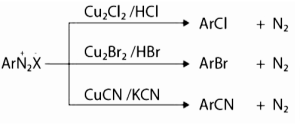
- Replacement by iodide ion:
It is not straightforward to insert iodine in the benzene ring directly. But when diazonium salt solution is treated with potassium iodide, iodobenzene is produced.
![]()
- Replacement by hydroxyl group:
When diazonium salt solution is heated to 283 K, it hydrolyzes to phenol.
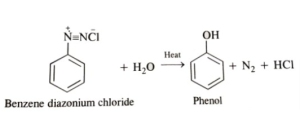
- Replacement by –NO2 group:
When heated with aqueous sodium nitrite solution and copper, diazonium fluoroborate becomes –NO2.
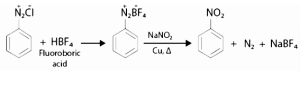
Diazonium Salts: Characteristics
- They have an ionic nature.
- They are soluble in water.
- Colourless crystalline solids, aryl diazonium salts are aryl diazonium salts.
- Although benzenediazonium chloride is soluble in water, it only reacts with it when it is heated.
- Water does not dissolve benzenediazonium fluoroborate. At room temperature, it is fairly stable.
Diazonium Salts’ Importance
- They are employed in the dye and pigment industries, as well as in the production of coloured fabrics.
- They are utilised in document reproduction because of their ability to break down when exposed to UV light.
- They can be used to make a wide range of chemical molecules, particularly aryl derivatives.
- For the preparation of aryl iodides and fluorides, direct halogenation is not an option. It is impossible to substitute a cyano group for the nucleophilic substitution of chlorine in chlorobenzene. Diazonium salts, on the other hand, can simply be used to make cyanobenzene.
- Direct substitution in benzene cannot be used to make substituted aromatic compounds. We make these compounds by replacing the diazo group in diazonium salts.
- They’re employed to introduce –F, –Br, –Cl, –I, –NO2, –OH, and –CN groups into the aromatic ring as intermediates.
Conclusion
- Although benzenediazonium chloride is soluble in water, it only reacts with it when it is heated.
- They are soluble in water.
- Colourless crystalline solids, aryl diazonium salts are aryl diazonium salts.
- They have an ionic nature.
- Water does not dissolve benzenediazonium fluoroborate. At room temperature, it is fairly stable.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




