The electrons in the outermost shell, known as valence electrons, are the only ones that can undergo changes in the atomic structure, regardless of the type of chemical bond that exists between atoms, such as an ionic, covalent, or metallic bond.
The most fundamental method would be to refer to an element’s atomic configuration and simply count the number of electrons in the outermost shell. However, this would be a very time-consuming task, as we may have to sift through textbooks in order to find configurations that we are unfamiliar with.
However, there is no need to be concerned because there is a much more straightforward method of determining this coveted number. In this more generalized approach, only one small resplendent rectangular sheet of paper — the periodic table — must be summoned in order to achieve success.
Simply consulting the periodic table and finding the element’s position within it is sufficient to determine the number of valence electrons present in an element.
Valence Electrons And The Periodic Table
The periodic table is a straightforward arrangement of all elements that have been discovered thus far. Atomic numbers or the number of protons or electrons that each element contains are listed from left to right in ascending order from the elements’ left to right positions.
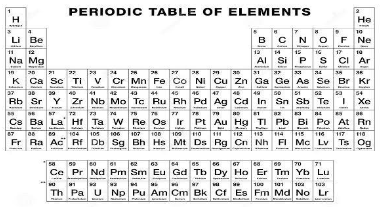
Four categories of elements are recognised: main group elements, transition elements, lanthanides, and actinides. The elements are classified as follows: In some circles, the latter two are referred to as “inner transition elements.”
There are a total of 18 columns in the table, which are formally known as groups, as well as rows, which are formally known as intervals. There are seven rows in the table above, and two rows below that distinguish between the rarer elements. The transition elements serve as a link between the elements in Groups 2 and 13, or they help to maintain the transition between them.
How To Find Valence Electrons ?
Even though the number of shells increases as we move down the group, the number of valence electrons remains constant as we move down.
However, while the number of valence electrons gradually increases over time by one, the number of shells remains constant. The number of shells encircling an element’s nucleus is indicated by the period number (also known as the row number, to remind you) in which it is found.
Valence Electrons Of Elements Other Than Transition Elements – The Main Group Elements
When it comes to electron valence, the period number indicates how many shells there are, whereas the group number indicates how many valence electrons there are in the outermost shell. To be more specific, the number in the ones’ position. This, however, is only true for the main group elements, which are the elements that are found in groups 1-2 and 13-18. The rule is inapplicable to the transition and inner transition elements for a variety of reasons (which we’ll discuss in more detail later).
With this method of simply referring to the periodic table and deducing the corresponding group number, the hassle and complexity that previously accompanied the arduous search for individual atomic configurations have been eliminated.
Conclusion
Valence electrons are those electrons that are found in the outermost shell of an atom and are responsible for its valence.The electrons in the outermost shell, known as valence electrons, are the only ones that can undergo changes in the atomic structure.
The most fundamental method would be to refer to an element’s atomic configuration and simply count the number of electrons in the outermost shell.The periodic table is a straightforward arrangement of all elements that have been discovered thus far. Atomic numbers or the number of protons or electrons that each element contains are listed from left to right in ascending order from the elements’ left to right positions.There are a total of 18 columns in the table, which are formally known as groups, as well as rows, which are formally known as intervals.
When it comes to electron valence, the period number indicates how many shells there are, whereas the group number indicates how many valence electrons there are in the outermost shell. To be more specific, the number in the ones’ position.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




