A crystal structure is formed by connecting atoms, groups of atoms, or molecules. This structure is created by the intrinsic nature of the constituent particles, which forms symmetric patterns. A structure’s unit cell is a small group of an atomic structure’s repeating pattern. A unit cell is a building block of a crystal structure which also describes the overall crystal structure and symmetry, and also atom positions and major axes. The length, borders of major axes, and angle between unit cells are all lattice constants or lattice parameters.
CRYSTAL STRUCTURE:
Atoms make a crystal structure. Points make a crystal lattice. A set of axes makes up a crystal system. In other terms, the structure is an array of atoms, ions, or molecules that are arranged in a specific order.
WHAT IS A UNIT CELL?
The smallest part of a crystal component is known as a unit cell. A crystal is made up of a group of atoms, ions, or molecules that are structured in a pure manner. The unit cells are organised in three-dimensional space, which describes the crystal’s bulk atom arrangement.
The following criteria are taken into account when identifying a unit cell in a crystalline structure.
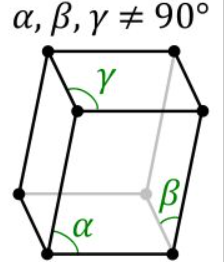
1.a, b, and c are the three edges.
2.The angles between these edges are α,β and γ
Angles between these edges can be different. The unit cells absorb and convey signals when high-energy electromagnetic waves travel through a crystal. These signals can be used to figure out what a unit cell’s form is. As a result, several unit cells’ mutual arrangements can be discovered, resulting in the expression of a crystal structure.
TYPE OF LATTICE STRUCTURE:
1.Primitive unit cells
Only the corner places are held by the constituent lattice points in this design.
2.Centered unit cells
The particles in this configuration occupy the centre as well as the corner places. They are divided into the following categories.
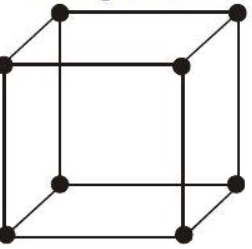
Body centered-cubic lattice structure (BCC):
The atomic planes or lattice planes of the respective atoms fall within the gaps of the lower planes of the respective atoms in this typical lattice structure. The unit cell in this type is formed like a cube. One atom is in direct contact with eight other atoms or lattice points, which is called direct contact. Vanadium, tungsten, and chromium all have this type of configuration.
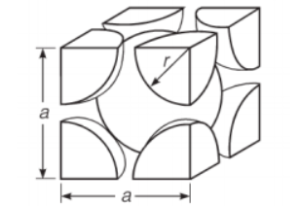
Face centered-cubic lattice structure:
In a stacking sequence, this lattice arrangement also packs the most atomic planes. In the underlying layer gaps, the second layer of atoms is predominantly layered following the HCP structure. The third layer, on the other hand, is found in the open spaces. Solid crystal lattice arrangements can be seen in metals like copper, lead, and nickel. This crystal structure has a coordination number of 12.
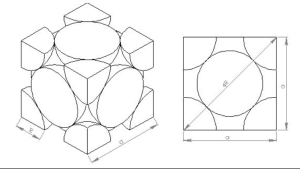
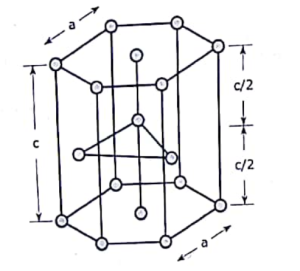
Hexagonal closed packed lattice structure(HCP):
The gaps created by superposed atoms or lattice points allow the underlying plane to fit into the higher one. A hexagonal closest packed (HCP) lattice structure is formed by the atomic levels being closely packed. Cobalt, zinc, titanium, and magnesium are examples of metals with this type of lattice arrangement. A point’s coordination number is 12 because it is related to 12 other points.
CRYSTAL SYSTEM:
One of the various classes of crystals, space groups, and lattices is referred to as a Crystal System. The lattice system and the crystal system are related in crystallography, although with a subtle distinction. Crystals and space groups are grouped into seven crystal systems based on their point groups.The Seven Crystal Systems is a method of categorising crystals based on their lattice and atomic structure. The atomic lattice is made up of a succession of atoms arranged in a symmetrical arrangement. The appearance and physical qualities of the stone may be determined with the help of the lattice. It’s feasible to figure out which crystal system they’re from. Crystals are considered to represent the element earth in the Cubic System. They are Seven Crystal Systems and are stated below with illustrated examples.
1.TRICLINIC SYSTEM:
All three axes are angled in the same direction and have the same length. The paired faces have diverse crystal shapes based on the three inclined angles. Labradorite, Amazonite, Kyanite, Rhodonite, Aventurine Feldspar, and Turquoise are examples of common Triclinic Systems.
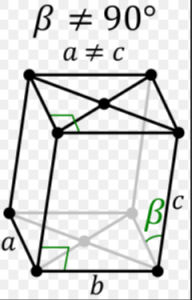
2.MONOCLINIC SYSTEM:
Each of the three axes has a different length. The first two are at right angles to one another, but the third is inclined. The inner structure is based on a parallelogram.
The following are examples of crystal shapes:
Prisms with sloped end faces and basal pinacoid
Monoclinic Crystals in Common Use:
Chrysocolla ,Chrysocolla, Diopside ,Azurite,Gypsum ,Epidote, Hiddenite,Kunzite, Lazulite .
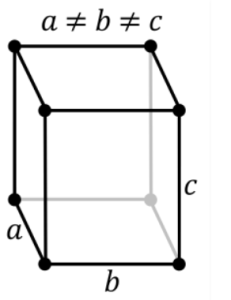
3. Orthorhombic System:
(System Rhombic) Three axes of varying lengths are positioned at right angles to one another.The interior structure is rhombic (diamond-shaped).
The following are examples of crystal shapes:
Pinacoids,Rhombic, Pyramids ,Double Pyramids
Common Orthorhombic Crystals :
Andalusite, (Chiastolite), Celestite, Chrysoberyl, Danburite, Dumortierite etc.
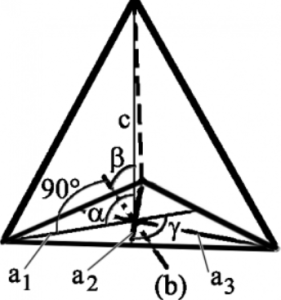
4.Trigonal System:
(Rhombohedral System) – This system’s axes and angles are similar to those of the Hexagonal System, and the two are frequently combined as Hexagonal. There will be six sides in the cross-section of a Hexagonal crystal. There will be three sides in the cross-section of a Trigonal crystal. The interior structure is triangular.
Common Trigonal Crystals:
Agate, Amethyst, Aventurine, Calcite, Carnelian etc.
5.Tetragonal System:
The primary axis is either longer or shorter, and all three intersect at right angles. The inner structure is rectangular.
Common Tetragonal Crystals:
Anatase, Apophyllite ,Chalcopyrite ,Rutile, Scapolite, Scheelite etc
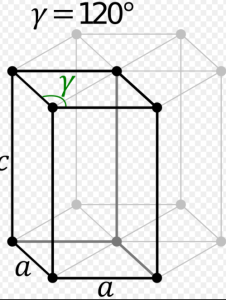
6. Hexagonal System:
Three of the four axes are in the same plane, have the same length, and cross at 60 degree angles. The fourth axis is shorter than the others and intersects them at right angles. The interior structure is hexagonal (6 sides).
Common Hexagonal Crystals
Apatite ,Aquamarine ,Beryl, Cancrinite, Emerald etc.
7. Cubic System:
The isometric system is another name for the isometric system. Each of the three axes is the same length and intersects at a right angle. The inner structure is square.
Common Cubic Crystals:
Diamond, Fluorite, Garnet, Spinel, Gold, Pyrite etc.
CONCLUSION:
Attaching atoms, groups of atoms, or molecules results in a crystal structure. The intrinsic nature of the constituent particles produces symmetric patterns, which results in this structure. The unit cell of a structure is a tiny group of a repeating pattern of the atomic structure. In this chapter we have discussed about crystal system, crystal structure, unit cell, the seven crystal system, type of lattice structure.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out