A coupling reaction in organic chemistry is a general term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M, where R is an organic fragment, M is the main group center reacts with an organic halide of the type R-X with the formation of a new carbon-carbon bond in the product R-R.
Richard F. Heck, Ei- Ichi Negishi, and Akira Suzuki were awarded the 2010 Nobel prize in chemistry for developing palladium-catalyzed cross-coupling reactions.
What is a coupling reaction?
The term coupling reaction refers to the class of organic reactions that involves the joining of two hydrocarbon fragments which are coupled with the help of a metal catalyst. Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. During the reaction, transmetalation occurs in which an alkyl group is transferred from one metal to another. The energy is transferred from the reactants’ side to the product’s side.
What happens in a coupling reaction?
- In a coupling reaction, an organometallic compound (R-M) reacts with an organic halide (R-X) to form a new carbon-carbon bond in the product (R-R).
- The common metal used in this field is pd, in addition to Zn, Ni, Cu, and Sn.
Types of coupling reaction:
Coupling reactions can be divided into two main classes, cross-couplings and homocoupling.
- Cross-coupling: In this type of reaction, two different molecules react to form one new molecule. It is also known as hetero coupling reactions.
Examples: Grignard reagent reaction, Suzuki reaction, Negishi coupling, and hiyama coupling.
- Homocoupling: In this type of reaction, two similar molecules are coupled together to form a new molecule.
Example: Wurtz reaction, pinacol coupling reaction.
Examples of cross-coupling reactions:
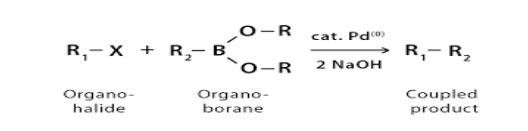
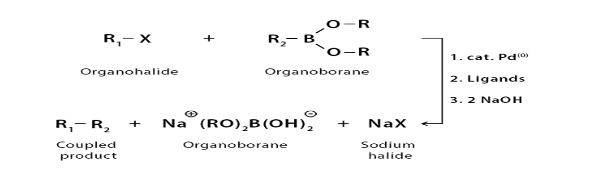
- Suzuki reaction: The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium complex.
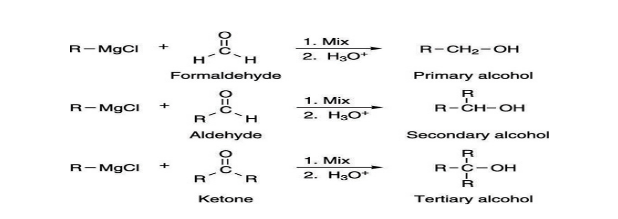
2. Grignard reagent reaction: The Grignard reaction is an organometallic chemical reaction in which alkyl, allyl, vinyl, or aryl magnesium halides are added to the carbonyl group in an aldehyde or ketone. This reaction is important for the formation of carbon-carbon bonds.
3. Negishi reaction: The Negishi coupling is a widely employed transition metal-catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon-carbon bonds in the process.
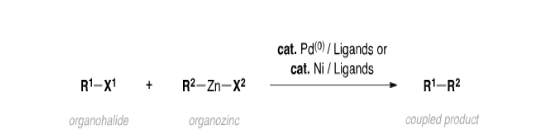
Examples of homocoupling reactions:
- Wurtz reaction: The Wurtz reaction, is a homocoupling reaction in organic chemistry, and organometallic chemistry. Where two alkyl halides are reacted with sodium metal in dry ether solution to form a higher alkane.
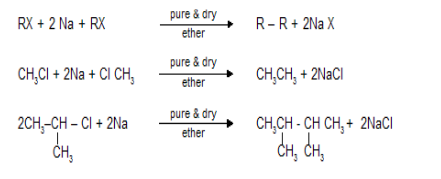
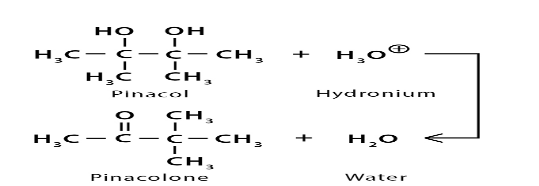
2.Pinacol coupling reaction: A pinacol coupling reaction is an organic reaction in which a carbon-carbon bond is formed between the carbonyl groups of an aldehyde or a ketone in presence of an electron donor in a free radical process. The reaction is also known as a vicinal diol.
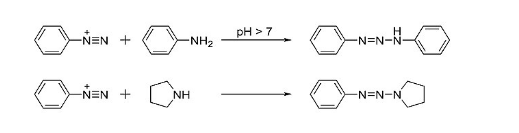
Azo coupling reaction:
Azo coupling is the most widely used industrial reaction in the production of dyes, lakes, and pigments. Aromatic diazonium ions act as electrophiles in coupling reactions with activated aromatics such as anilines and phenols. Azo coupling is an organic reaction between a diazonium compound and another aromatic compound that produces an azo compound. In this electrophilic aromatic substitution reaction, the aryl diazonium cation is the electrophile and the activated arene is a nucleophile.
Applications of coupling reactions:
The following are some of the most important applications of coupling reactions:
- The coupling reaction is used in various applications, including the synthesis of new and complex conjugated polymers and natural products. It is also used in the manufacture of new pharmaceutical drugs.
- Many conjugated polymers are made with the aid of a metal catalyst in coupling reactions.
- Several pharmaceutical drugs are synthesized using coupling reactions.
- The Suzuki coupling reaction is often used to make complex compounds synthetically. It is, for example, used in the manufacture of caparratriene, a highly effective leukemia medication.
- Cross-coupling reactions are used to make benzylisoquinoline alkaloids.
Conclusion:
Coupling reaction refers to the class of organic reactions that involves the joining of two hydrocarbon fragments which are coupled with the help of a metal catalyst. Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. In a coupling reaction, an organometallic compound (R-M) reacts with an organic halide (R-X) to form a new carbon-carbon bond in the product (R-R). The common metal used in this field is pd, in addition to Zn, Ni, Cu, and Sn. The most common type of reaction is the cross-coupling reaction.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




