It is known as geometric isomerism or configurational isomerism in chemistry. It is a term that refers to the spatial arrangement of atoms within molecules, and it is used to describe the spatial arrangement of atoms in molecules. Trans and trans- are prefixes derived from Latin, which means “this side of” and “other side of” in English, respectively. In the context of chemistry, cis denotes that the functional groups (substituents) are on the same side of a plane, but trans denotes that they are on the other (transverse) side of a plane, and vice versa. Cis–trans isomers are stereoisomers, which are pairs of molecules that have the same formula but whose functional groups are oriented in different directions in three-dimensional space. Cis–trans isomers are stereoisomers because they have the same formula but have different orientations in three-dimensional space. When it comes to E–Z isomerism, which is an absolute stereochemical description, cis-trans nomenclature does not necessarily match to it. A common feature of trans–trans stereoisomer compounds is that they contain double bonds that do not rotate. In addition, they may contain ring structures in which the rotation of the bonds is restricted or prevented. Cis and trans isomers can be found in both organic and inorganic compounds, as well as in coordination complexes. In cases of conformational isomerism when the two geometric forms are easily interconvertible, such as most open-chain single-bonded structures, the descriptors “cis” and “trans” are not employed; instead, the terms “syn” and “anti” are used to describe the two geometric forms.
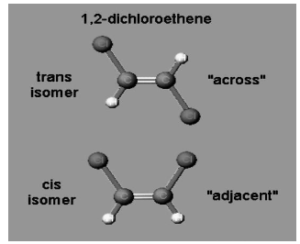
1,2- Dichloroethene
The chlorine atoms in the example on the left can be either opposite or across from one another, in which case the isomer is referred to as the “trans” isomer. If the chlorine atoms are located next to or next to one another, the isomer is referred to as “cis.”
There can’t be any geometric isomers if one of the carbons in a double bond has two identical groups, such as two H’s or two Cl’s or two CH3‘s, for example.
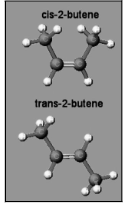
2-Butene
Take, for example, the longest chain that has a double bond: In the case of a cis alkene, two groups (attached to the carbons of the double bond) are on the same side of the double bond as the double bond itself. An enantiomer is a trans alkene if the two groups on either side of the double bond are on opposite sides of the double bond. One or more of the “groups” may or may not be a part of the longest chain, depending on the circumstances. For example, the “group” represented by the letter “M” is a methyl – yet it is actually a component of the longest chain.
It is customary to refer to this chemical as 1,2-dimethylethene, which is incorrect. Look at all of the carbons to see which one has the longest continuous chain – the root is butene, which has four carbons.
Geometric isomerism (in the cis and trans directions)
These isomers can be found in molecules if there is a restriction on rotation at some point in the molecule. When teaching organic chemistry at an introductory level, examples usually just contain the carbon-carbon double bond — which is what this article will focus on. Consider what happens in molecules where there is unconstrained rotation about carbon bonds – in other words, where the carbon-carbon bonds are all single – and what happens in molecules where there is restricted rotation about carbon bonds. 1,2-Dichloroethane can be found in two different configurations, as depicted in the following diagram.
Identify the probability of geometric Isomerism in a given situation
It goes without saying that restricted rotation must exist someplace in the molecule. Compounds with a carbon-carbon double bond have a restricted rotation due to this bond. Although other types of compounds may also have restricted rotation, we are focussing on the scenario that you are most likely to encounter when learning about geometric isomers for the first time here. When dealing with a carbon-carbon double bond, it is important to consider the potential of geometric isomers carefully.
Geometric Isomers can only be obtained if the following conditions are met:
Two separate groups on the bond’s left-hand end and two different groups on the bond’s right-hand end are required for restricted rotation (which is frequently a carbon-carbon double bond for introductory reasons). Whatever the case, it makes no difference if the left-hand groups are the same as the right-hand groups.
Property Dissimilarities
The physical properties of the cis-trans isomers of a compound differ in many cases. Changes in the dipole moment of the molecule or variances in the spatial arrangement of atoms might cause these differences. Some of these distinctions are highlighted in the table below.
- The cis isomer of pent-2-ene has a boiling point of 37°C, while the trans isomer has a boiling temperature of 36°C. Because the bond polarity is low, the change is minor.
- The cis isomer’s boiling point is 60.3°C, while the trans isomer’s is 47.5°C, due to the polar nature of the bonds in 1,2-dichloroethylene.
- Because of the differences in their characteristics, the cis and trans isomers of butenedioic acid have extremely distinct reactivities. The cis isomer of maleic acid is maleic acid, while the trans isomer is fumaric acid.
- The cis-trans isomers of elaidic acid and oleic acid are elaidic acid and oleic acid, respectively. At room temperature, the former is solid (melting point = 43°C), whereas the latter is liquid (melting point = 13.4°C).
As a result, it’s worth noting that trans isomers have higher melting points than their cis counterparts. They also have a decreased solubility in solvents that are naturally inert. It’s been suggested that trans isomers’ densities are lower than those of cis isomers.
The individual bond dipole moments of trans isomers, in general, cancel each other out because they are on opposing sides. The steric interaction of the substituents explains why the cis isomers of acyclic systems are less stable than the trans variation.
Conclusion
The atoms of cis-trans isomers are arranged differently in three-dimensional space. In organic chemistry, cis isomers have functional groups on the same side of the carbon chain, while trans isomers have functional groups on opposing sides.
Both organic and inorganic compounds can exhibit this isomerism. The prefixes “cis” and “trans” mean “this side of” and “other side of”, respectively. cis-trans isomers of various coordination complexes.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out