Some alkene compounds display a particular sort of isomerism as a result of the double bond. Rotation around a single bond is easy, but it is difficult to rotate around a double bond. Because the electrons overlap both above and below the plane of the atoms, the pi bond inhibits rotation.
Two boards are fastened together with one nail in a single bond. Two boards are fastened together with two nails in a double bond. The boards can be twisted in the first example, but they cannot be twisted in the second.
Compounds having varying spatial configurations of groups linked to the carbons of a double bond are known as geometric isomers. The carbon-carbon double bond is securely anchored in alkenes.
A guiding criterion in the search for geometric isomers is that there must be TWO DIFFERENT “GROUPS” ON EACH CARBON OF THE DOUBLE BOND. Hydrogen, alkyls, halogens, and other elements can form a “group.”
The atoms in isomers of substances are arranged differently. The arrangement of the atoms in isomer compounds differs from identical compounds. Take a look at the sample below.
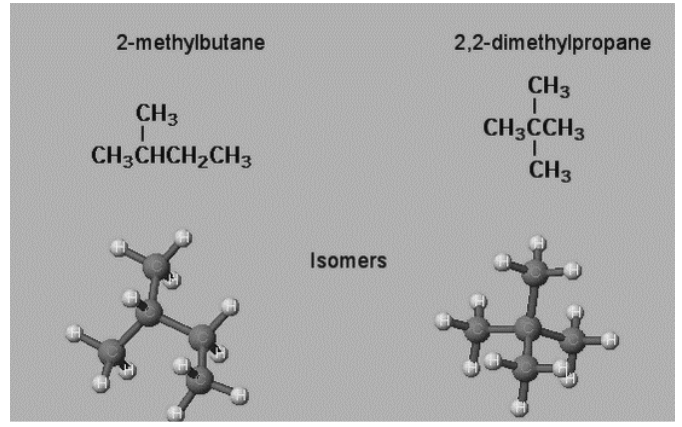
C5H12 is the same number of atoms in both molecules. Because the root of the left molecule has four carbons and one branch, they are isomers. The root of the right molecule has three carbons and two branches. Because they contain the same number of atoms but distinct configurations of those atoms, they are isomers.
Compounds that are fully distinct: If the quantity of elements in each compound differs, the two compounds are just completely different. A simple count of the atoms reveals that they are distinct.
1,2-Dichlorethene
The chlorine atoms in the example on the left might be opposite or across from each other, resulting in the “trans” isomer. The isomer is called ” cis” when the chlorine atoms are close to or next to one other.
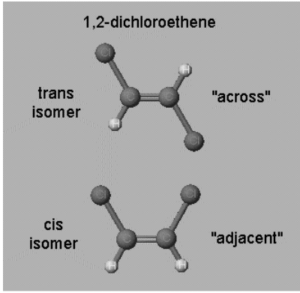
There can’t be geometric isomers if one of the double bond’s carbons has two identical groups, such as two H’s, two Cl’s, or two CH3‘s.
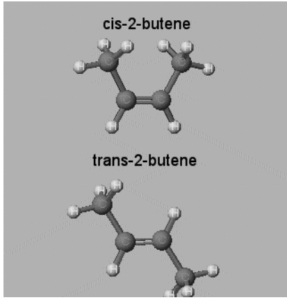
2-Butene
Take a look at the longest chain with a double bond: The isomer is a cis alkene if two groups (connected to the carbons of the double bond) are on the same side of the double bond. The isomer is a trans alkene if the two groups are on opposing sides of the double bond. The longest chain may or may not include one or more of the “groups.”
Isomers of Alkanes
A homologous series is a collection of compounds in which each member differs from the previous by one CH2 unit. As an example, the series CH4,C2H6,C3H8. . . CnH2n+2, is a homologous series.
It’s critical that you memorise the names of the first ten straight-chain alkanes (from CH4 to C10H22). When you start learning how to derive the systematic names of a wide range of organic compounds, you’ll use these names a lot. You don’t have to recall how many isomers are feasible for alkanes with more than seven carbon atoms. When such knowledge is required, it can be found in reference books. When sketching isomers, take care not to trick yourself into believing you can draw more isomers than you actually can. Remember that each isomer can be drawn in a variety of ways, and you may unintentionally count the same isomer many times.
Methane (CH4), ethane (C2H6), and propane (C3H8) are the first in a series of compounds that differ by one carbon atom and two hydrogen atoms—a CH2 unit—between any two members in the sequence. Starting with C3H8, a CH2 units are added in each stage as the series progresses. A homologous series is a set of molecules in which adjacent members differ by a specified factor (in this case, a CH2 group).
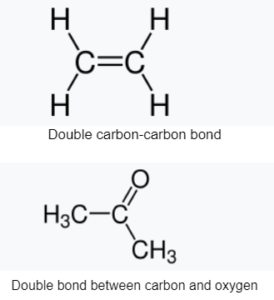
Double Bond
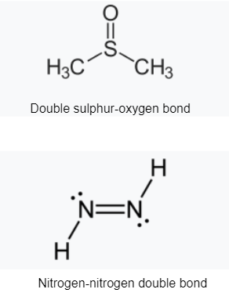
A double bond is a covalent link between two atoms that involves four bonding electrons rather than the two seen in a single bond. Alkenes, for example, are made up of double bonds between two carbon atoms. A carbonyl group between a carbon atom and an oxygen atom is an example of a double bond between two distinct atoms. Azole compounds (N=N), imines (C=N), and sulfoxides (S=O) are all examples of common double bonds. A double bond is represented in a skeleton formula by two parallel lines (=) connecting the two linked atoms; the equals sign is used typographically for this. Russian chemist Alexander Butlerov was the first to use the term “double bond” in chemical notation.
Cis & Trans Isomers of Alkanes
Take a look at the longest chain with a double bond: The isomer is a cis alkene if two groups (connected to the carbons of the double bond) are on the same side of the double bond. The isomer is a trans alkene if the two groups are on opposing sides of the double bond.
Because the atoms are arranged differently, many organic molecules with identical components will have vastly different physical and chemical characteristics. In a formula, isomers and identical compounds both have the same number of each type of ingredient. This can be proven with a simple count.
Conclusion
In cyclic molecules, cis-trans isomerism also occurs. Groups in ring structures cannot spin around any of the ring carbon–carbon links. As a result, groups might be on the same side of the ring (cis) or on the opposite side (trans) (trans). For our purposes, all cycloalkanes are represented as planar structures, with the locations of the groups indicated above or below the plane of the ring.

Cis-1,2-dimethylcyclopropane
Trans-1,2-dimethylcyclopropane.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






