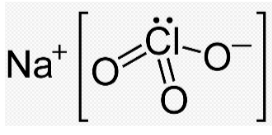
When sodium chloride reacts with oxygen it produces an inorganic substance having the chemical formula NaClO3, and it is found in nature in White crystalline powder that is readily soluble in water, it has a sweet taste to it. It has a hygroscopic property. It decomposes at temperatures exceeding 300 degrees Celsius, releasing oxygen and leaving sodium chloride behind. The product is produced in large quantities, with several hundred million tons produced annually, mostly for use in bleaching pulp to generate high-brightness paper. It is possible to chlorinate aromatic compounds without the need of organic solvents by combining sodium chlorate with hydrochloric acid (or with sulfuric acid and sodium chloride, the reaction of which yields HCl). If the pH is high enough, it will oxidize the HCl to produce either HOCl or Cl2 (depending on the pH), which are the active chlorinating agents.
Reaction between Sodium chloride and oxygen
One of its physical characteristics is that it is white in color and that it is crystalline in nature, meaning that it dissolves quickly in water. In nature, it has been observed to be hygroscopic (capable of absorbing moisture from the surrounding air). It decomposes at a temperature of 573 degrees Kelvin, releasing O2 and leaving behind NaCl. The production of sodium chlorate is substantial each year, with the majority of it going into the oxidizing mash, which is used to produce high-quality paper.
Preparation of Sodium Chlorate (Sodium chloride + oxygen)
In order to make sodium chlorate, salty water is electrolyzed (sodium chloride and water)
NaCl + 3 H2O + 6 e– → NaClO3 + 3 H2
An exothermic reaction is at work here. It’s a series of events. The technique can also be affected by changes in pH and temperature.
Chlorine gas (Cl2) is stored at the anode and hydrogen gas (H2) is stored at the cathode in the manufacture of sodium chlorate. The hypochlorite anion group formed as a result of chlorine’s hydrolysis in the cell yields sodium chlorate.
There are a lot of crystals in sodium chlorate. Cell liquor is a term used to describe the hydrolyzed solution. Remove the solution from the device. After they’ve been cleaned and dried, the crystals can be stored in a dry location. It can be sold in crystals or as a liquid, depending on the intended usage. To produce 1 mol of chlorate, 6 mol of chloride must be discharged, regardless of the reaction pathway. In contrast, the anodic oxidation method requires 50% more electricity. Autoxidation is hence the preferred mechanism for industrial cells. In order to minimize loss reactions, chlorate production at the anode is considered to be a loss reaction.
Physical properties of Sodium chlorate
There are a lot of inorganic salts out there, and sodium chlorate has a lot of the same physical properties. Some of them are on the list below.
- It doesn’t have any smell.
- Its colour ranges from a light yellow to a white solid that looks like crystals.
- It is very easy to dissolve in water, and it is heavier than water, so it is very easy to drink. Thus, it can sink and break up quickly.
- However, even though it isn’t explosive on its own, water can start a very powerful fire. It causes a lot of heat to be released. Molecules can cause a powerful oxidising reaction even if only 30% of them are in the water. This is because of their own properties.
- It weighs 2.49 g/cm.
- sodium chlorate can boil at 300oC and melt at 248oC.
- There are some organic solvents that it can also be used with, like glycerol and methanol. It is also a little bit soluble in acetone.
- It has a cube-shaped crystal structure.
Uses of Sodium Chlorate
Sodium chlorate is utilised in a wide variety of applications. Several common applications are listed here.
- The primary commercial application of sodium chlorate is in the production of chlorine dioxide (ClO2). The primary application of ClO2, which accounts for approximately 95% of chlorate use, is in pulp bleaching.
- Herbicides, explosives, colours, matches, inks, beautifiers, medications, defoliants, paper, and calfskin are all made with it.
- It is used as a dying mash in the papermaking process.
- Utilised in the Solvay process, which eliminates salt and H2SO4, with the addition of CH3OH as a lessening specialist.
- It is utilised as an oxidising expert and an oxidising and bleaching operator in large-scale dye manufacturing processes.
- It is used in the medical field to prepare a variety of medications.
- It is utilised in the manufacture of fertilisers and explosives.
CONCLUSION
When aqueous sodium chloride is electrolyzed, sodium hypochlorite, or NaOCl, is formed, which is a sodium, oxygen, and chlorine compound used in large quantities in household chlorine bleach. Sodium hypochlorite is also used as an antibiotic and fungicide in certain pharmaceutical formulations, as well as an industrial bleach for paper pulp and textiles and water chlorination. It is a highly unstable chemical that can only be found in aqueous solution. Other notable commercial applications of sodium chloride include electrolytic decomposition for the generation of chlorine and sodium hydroxide, as well as the Solvay method for the manufacturing of sodium carbonate (Na2CO3).
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out