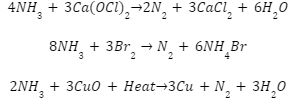Ammonia, a colourless gas with an unique odour, is a chemical building block that is used to make a variety of daily things. It can be found in the air, soil, and water, as well as in plants and animals, even humans. The first element in the periodic table’s group 15 is nitrogen. Nitrogen is found in a variety of chemicals, including nitric acid, nitrogen dioxide, ammonia, and ammonium hydroxide.
Ammonia
Ammonia is a nitrogen and hydrogen based chemical. It has one nitrogen atom and three hydrogen atoms. NH3 is its chemical formula. The natural breakdown of animal and plant bodies generates ammonia since the nitrogen compounds present in them decompose as they die or decay, resulting in ammonia. Ammonia can also be found in the soil as ammonium salt.
Ammonia is a fundamental component of a wide range of chemicals and products. Ammonia is used in a variety of products, including fertilisers, polymers, household cleaning agents, and as a refrigerant gas. Its applications can also be traced back to the pharmaceutical industry. But it is Ammonia’s ability to synthesize Ammonium Nitrate and Ammonium Sulfate, that are the most essential components of artificial fertilisers today, and the special ingredient for plant growth and huge production around the world, that makes it the most important molecule.
Ammonia is made in small amounts by the decomposition of urea, a nitrogenous organic substance. Laboratory Preparation is another name for this procedure. The reaction begins with the degradation of urea with water to produce ammonium carbonate, which then decomposes and undergoes an equilibrium reaction to produce ammonia while simultaneously releasing gases such as hydrogen and carbon dioxide. The following is a representation of the equation:
NH2CONH2+2H2O↔NH42CO32NH3+H2O+CO2
Physical Properties of Ammonia
Ammonia is a colourless gas with a distinct pungent odour referred to as ammoniacal odour.
Since it is lighter than air, it is gathered via air displacement downward.
As inhaled quickly, it causes tears to well up in the eyes.
Whenever a pressure of 8 to 10 atmospheres is applied, it will easily liquefy at ambient temperature.
Under one atmosphere pressure, liquid ammonia boils at – 33.5℃. It has a high enthalpy of evaporation (1370J/g) and is hence employed in ice-making equipment and refrigeration systems.
It has a high solubility in water. One litre of water dissolves around 1300 litres of ammonia gas. Because of its high water solubility, ammonia gas cannot be collected on water.
Chemical Properties of Ammonia
Basic nature
Ammonia is extremely water soluble. Its aqueous solution is slightly basic because to the formation of OH- ions.
NH3+H2O ↔NH4OH ↔NH4++OH-
Combustion
Ammonia is neither a fuel nor a supporter of a fuel. In the presence of oxygen, meanwhile, it produces dinitrogen and water.
4NH3+3O2→2N2+6H2O
Chemical reactivity of ammonia with oxygen
Oxidation
Ammonia is converted to dinitrogen gas whenever it passes via a solution of calcium hypochlorite (bleaching powder), bromine water, or heated copper oxide.
When ammonia is fed over Pt/Rh gauze at 500K under a pressure of 9 bar with an excess of air, it is converted to nitric oxide.
Ostwald’s technique uses this reaction as the starting point for making nitric acid.
Uses of Ammonia
The following are among the several uses for ammonia:
In the production of nitric acid by Ostwald.
The Solvay process is used in the production of sodium carbonate.
It’s used to make rayon and urea, among other products.
Fertilisers including ammonium sulphate, ammonium nitrate, urea, diammonium phosphate, and others are manufactured.
As a refrigerant in ice plants.
It can be used to clean furniture and glass surfaces.
It is used as a reagent and a solvent in the laboratory.
Applications of Ammonia
The most well-known and studied nonaqueous ionising solvent is liquid ammonia. Its capacity to dissolve alkali metals into vividly coloured, electrically conductive solutions containing solvated electrons is its most notable feature. Aside from these unusual solutions, much of the chemistry of liquid ammonia can be classed by analogy with related aqueous processes.
Conclusion
Ammonia is a nitrogen-hydrogen chemical with the formula NH3. Ammonia is a colourless gas with a pungent odour. It is a stable binary hydride and the lowest pictogen hydride. It is a frequent nitrogenous waste among aquatic organisms, and it contributes significantly to terrestrial organisms’ nutritional needs by serving as a precursor to 45%of the world’s food and fertilisers. Ammonia is also employed in many commercial cleaning products and is a building block for the production of many pharmaceutical medicines, either directly or indirectly. It is mostly gathered by air and water displacement downward.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out