Acetic acid (CH3COOH), also known as Ethanoic acid, is the most important Carboxylic acid. A vinegar is a dilute (approximately 5% by volume) solution of acetic acid produced by fermentation and oxidation of natural carbohydrates; acetate is an acetic acid salt, ester, or acylal. Acetic acid is used in the manufacturing of metal acetates, which are used in some printing processes; vinyl acetate, which is used in the production of plastics; cellulose acetate, which is used in the production of photographic films and textiles; and volatile organic esters (such as ethyl and butyl acetates), which are widely used as solvents for resins, paints, and lacquers.
Acetic acid is a critical metabolic intermediate that occurs naturally in body fluids and plant juices. On an industrial scale, acetic acid has been produced by air oxidation of acetaldehyde, ethanol (ethyl alcohol), and butane and butene. Today, Acetic acid is produced using a process developed in the 1960s by the chemical company Monsanto, which involves a rhodium-iodine catalysed carbonylation of methanol (methyl alcohol). Pure acetic acid, also known as glacial acetic acid, is a corrosive, colourless liquid that is completely miscible with water (boiling point 117.9 °C [244.2 °F]; melting point 16.6 °C [61.9 °F]).
Chemical Reactivity of Acetic acid with Oxygen
Using temperature-programmed desorption (TPD) and synchrotron radiation photoelectron spectroscopy, we investigated the role of co adsorbed atomic oxygen during the decomposition of acetic acid on Pt(111) (SRPES). Identification of desorbing products and surface species formed during acetic acid decomposition on Pt(111) and oxygen pre-exposed p(2 2)–O/Pt has revealed reaction mechanisms (111). Acetate and molecularly adsorbed acetic acid are formed on both samples during acetic acid adsorption at 150 K. However, surface acetyl is identified as the dominant species on p(2 2)–O/Pt(111).
At 222 K, the major decomposition channel for acetate and acetic acid involves the formation of ketene and acetaldehyde, which is unaffected by co adsorbed oxygen. On both samples, partial decomposition of acetaldehyde yielded ethylene, ethylidene, ethylidyne, and small amounts of CO and methoxy. Decomposition of acetate on Pt(111) above 222 K produces acetic acid, hydrogen, methane, and CO.
In contrast, the species desorbing from p(2 2)–O/Pt(111) are acetyl decomposition products. At 300 and 450 K, acetyl reacts with atomic oxygen and surface hydroxyl groups to produce methanol and acetic anhydride, respectively, and methane and CO2 at 390 K. Acetic acid decomposition on Pt(111) and p(2 2)–O/Pt(111) results in surface carbon from ethylidyne decomposition and partial C–C bond cleavage in the acetyl species.
Acetic Acid Structure
Acetic acid is a carboxylic acid with a complex structure. Because it contains the carboxyl function group, it is classified as a carboxylic acid. A carboxyl group is made up of a carbon-oxygen double bond as well as an OH group.
Take note of the carboxyl group in the acetic acid image. The carboxyl group (on the right) is bonded to a group of atoms (on the left) (one carbon single bonded to three different hydrogen atoms).
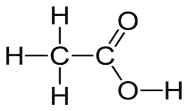
Acetic Acid Reactions
A proton donor is a substance such as acid. Acetic acid’s carboxyl functional group (-COOH) contains a hydrogen capable of donating an H+ ion. This H+ ion is also referred to as a proton.
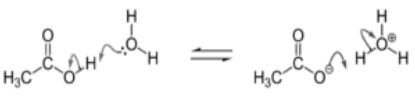
Proton Donation in Acids
Carboxylic acids react with carbonates and bicarbonates to produce salts, water, and carbon dioxide.
A classic example of this type of reaction can be found in school experiments involving the mixing of vinegar (which contains acetic acid) and baking soda (which contains sodium bicarbonate). As shown, the reaction produces sodium acetate, carbon dioxide, and water:
CH3COOH + NaHCO3 → NaC2H3O2
Acetic acid will decompose into carbon dioxide and methane at temperatures above 440 degrees Celsius, or into ethenone and water as shown:
CH3COOH → CO2 + CH4
CH3COOH → H2C = C = O + H2O
Conclusion
Acetic acid , also known as ethanoic acid, is the most important carboxylic acid. A vinegar is a dilute solution of acetic acid produced by fermentation and oxidation of natural carbohydrates; acetate is an acetic acid salt, ester, or acylal. Acetic acid is used in the manufacturing of metal acetates, which are used in some printing processes; vinyl acetate, which is used in the production of plastics; cellulose acetate, which is used in the production of photographic films and textiles; and volatile organic esters (such as ethyl and butyl acetates), which are widely used as solvents for resins, paints, and lacquers. Pure acetic acid, also known as glacial acetic acid, is a corrosive, colourless liquid that is completely miscible with water. Acetic acid is a carboxylic acid with a complex structure.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




