An oxidising agent is a chemical that may oxidise other compounds in chemistry. You could be perplexed by the term “oxidation.” A material can receive electrons from other substances, known as oxidation. Hydrogen peroxide, oxygen, Halogens, ozone, hexavalent chromium compounds, and other oxidising agents are most often used.
Potassium compounds are widely used and have several applications. Glass has been made with potassium and other potassium compounds for ages. Today, fertilisers are made from 95% of all potassium compounds collected worldwide.
An inorganic chemical compound with the chemical formula for K2Cr2O7 is an orange crystalline solid. It’s a powerful oxidiser that’s also water-soluble. It is employed to prepare chrome alum, chrome yellow, and chrome red for volumetric quantification of ferrous salts, iodides, and sulphides.
What is Potassium Dichromate?
Potassium dichromate is a hexavalent chromium compound used as an inorganic chemical reagent. K2Cr2O7 is the chemical formula for potassium dichromate. As a traditional oxidising agent, it’s employed in various industrial applications and labs. Potassium dichromate is very poisonous and detrimental to the skin and body since it is hexavalent. The crystalline ionic solid potassium dichromate has a vivid red-orange colour.
Compared to most industry-relevant sodium dichromate salts, potassium dichromate is more common in laboratory research since it is not deliquescent (it tends to absorb air moisture and dissolve in it). Potassium dichromate is formed when potassium chloride reacts with sodium dichromate.
It has a vivid red-orange colour and is a crystalline ionic solid. It has no odour and, is insoluble in acetone and alcohol, dissolves in water.
It’s a common ingredient in potassium chrome alum and leather tanning. It’s widely utilised as an oxidiser in various industries and laboratories. It’s extremely corrosive and non-flammable. The following steps are used to make potassium dichromate:
- The chromite ore or FeCr2O4 reacts with sodium carbonate or Na2CO3 to form sodium chromate.
4FeO.Cr2O3 + 7O2+ 8Na2CO3 → 8Na2CrO4 + 2Fe2O3 + 8CO2
- This step involves reaction of sodium chromate( Na2CrO4), with concentrated sulphuric acid(H2SO4) to get sodium dichromate solution.
2Na2CrO4 + 2H+ → Na2Cr2O7 + 2Na+ + H2O
We also get Sodium ions from this reaction.
- In the final step sodium dichromate solution is mixed with potassium chloride or KCl to form potassium dichromate or K2Cr2O7.
Na2Cr2O7 + 2KCl → K2Cr2O7 + 2NaCl
The Structure of Potassium Dichromate (K2Cr2O7)
Potassium Dichromate has a molar mass of 294.185 g/mol and a chemical formula of K2Cr2O7. It’s an ionic molecule made from two potassium ions and the negatively charged dichromate ion. In this, two hexavalent chromium atoms are connected to three oxygen atoms plus a bridging oxygen atom.
Properties of Potassium Dichromate
- The crystal structure of K2Cr2O7 is reddish-orange.
- This chemical is 49 grams per litre soluble in water at 0°C.
- Alcohol and acetone do not dissolve this chemical.
- 219 Joules per mole are the heat capacity of K2Cr2O7.
- This compound’s molar entropy is 291.2 joules per kelvin mole, the usual value.
Physical Properties of Potassium Dichromate
At room temperature, potassium dichromate is solid with an orange-red crystal structure.
In its natural state, potassium dichromate has no odour.
In nature, potassium dichromate is very corrosive and non-combustible.
Potassium dichromate melts at 398 degrees Celsius and boils at 500 degrees Celsius. Potassium dichromate decomposes when it is heated to a certain temperature.
At different temperatures, the solubility of potassium dichromate varies. It is extremely soluble in water at high temperatures, which indicates it is highly soluble in water. However, alcohol does not dissolve potassium dichromate as efficiently as acetone does.
Potassium dichromate has a 1.738 refractive index. The chromium ion has a tetrahedral coordination geometry, while potassium dichromate has a triclinic crystalline structure.
Chemical Properties of Potassium Dichromate
- Heat produces chromate as well as oxygen gas when potassium dichromate is heated.
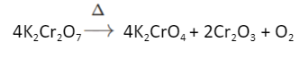
Potassium dichromate’s reaction with alkalis: When potassium dichromate reacts with alkalis, it loses its orange-red colour and becomes yellow.
K2Cr2O7 + 2KOH → 2K2CrO4 + H2O (here K2Cr2O7 is orange and K2CrO4 is yellow)
- Potassium dichromate has oxidising characteristics. The oxidising agent potassium dichromate is very effective. Potassium dichromate can be used as an oxidiser in the following applications:
– One mole of potassium dichromate yields three moles of oxygen gas when treated with dilute sulphuric acid.
K2Cr2O7 + 4 dil.H2SO4 → K2SO4 + Cr2 (SO4)3 + 4H2O + 3(O)
– Iodine gas is released when potassium dichromate reacts with KI.
K2Cr2O7 + 7H2SO4 + KI → Cr2(SO4 )3+ 3I2 + 4K2SO4 + 7H2O
Potassium dichromate interacts with concentrated sulphuric acid to produce chromyl chloride vapours, reddish-brown. The Chromyl Chloride Test refers to this reaction.
K2Cr2O7 + 4KCl + 6H2SO4 → 2CrO2Cl2 + 6 KHSO4 + 3H2O ( CrO2Cl2 is Chromyl Chloride)
Uses of potassium Dichromate
Potassium dichromate is an oxidising specialist in research institutions and industries for a wide range of reactions.
It serves as an antecedent for potassium chrome alum in the chrome tanning of calfskin.
In volumetric exams, it is employed.
It’s used for calico printing and colouring.
The production of potassium chrome alum and the tanning of cowhide are two of the most common uses for potassium dichromate.
- To harden gelatin film in photography, potassium dichromate (K2Cr2O7) is used as an oxidising specialist with solid mineral acid.
It’s used to make chromic corrosives (such as chromium (VI) compounds, sodium dichromate, and chromium trioxide), which are used to clean dishes.
Effects of Potassium Dichromate on Health
Potassium dichromate can lead to chronic infections in the hands and lower arms, such as chromium dermatitis.
Bunnies, rodents, and other animals can be harmed by potassium dichromate.
It’s also bad for amphibians, and it’s a big problem for the environment.
Potassium dichromate is toxic, and its presence can lead to a variety of eye problems, including blindness.
It has the potential to induce genetic damage, impaired ripeness, and danger to unborn children.
Conclusion
As potassium dichromate is a hexavalent chromium compound, it is poisonous and cancer-causing. Potassium dichromate is corrosive by nature, causing severe eye and skin irritation, burning sensations, and even blindness. It impairs reproductive health and works as a mutagenic agent, altering genetic material and harming unborn infants.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



