When we do a method to check primary amines, carbylamine is generated. When the amine is introduced to the reaction, several chemicals are generated in the Carbylamine chemical test.
Isocyanides or carbylamine’s are formed by heating aliphatic and aromatic primary amines with chloroform and ethanolic potassium hydroxide, resulting in an unpleasant odor. Secondary and tertiary amines do not undergo this reaction.
This is known as the isocyanide test or the carbylamine reaction, and it is used to identify primary amines. Hoffmann’s isocyanide synthesis is another name for the carbylamine reaction.
Ph−NH2+CHCl3+alc. KOH → ph−N≡C+KCl+H2O.
Isocyanide Test by Hoffmann
Because it is only effective for primary amines, the carbylamine reaction could be used as a chemical test to check for their existence. When employed as a test, the carbylamine’s reaction is also known as Hofmann’s isocyanide test.
This process involves heating the test material with chloroform and alcoholic potassium hydroxide. Isocyanide (carbylamine) will be generated in the existence of primary amine, and its terrible odor will readily identify it.
The Hofmann isocyanide test does not produce a foul odor when tested with secondary and tertiary amines because secondary and tertiary amines do not undergo the carbylamine reaction.
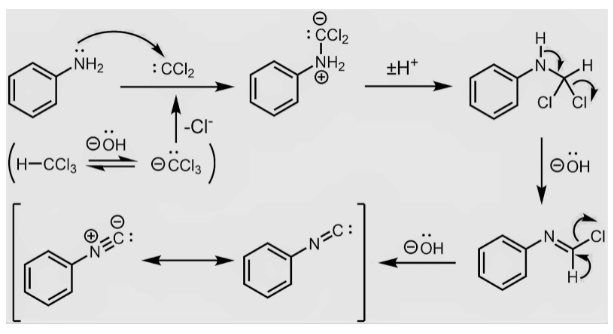
Mechanism of Carbylamine Reaction
- Dehydrogenation is removal of hydrogen halide from specific substrate of chloroform is the first step, which yields Dichlorocarbene as an intermediate. Dichlorocarbene is a highly reactive intermediate.
- The electrophilic dichlorocarbene targets the main amine’s nucleophilic nitrogen.
- The hydrochloric acid is then eliminated, resulting in the creation of isonitrile.
R-NH2 + CHCL3 + 3KOH R-NC + 3KCl + 3H20
CH3-NH2 + CHCl3 + 3KOH (alc) CH3-NC + 3KCl + 3H2O
Why does the Carbylamine Reaction Mechanism described above fail to function in Secondary or Tertiary Amines?
The Carbylamine Reaction Mechanism, as we all know, is frequently employed to detect chloroform and even the primary amine. This carbylamine’s test is used to distinguish between the major amine and the high and low amines. Isopropyl amine produces a positive carbylamine test.
In practice, that was one of the best carbylamine’s reaction examples of the Alpha reaction, as the carbanion was formed first, then the chloride Ion was lost to form the most effective dichlorocarbene.
Following that, chlorine atom atoms will be eliminated using HCl. As a result, hydrogen is removed from nitrogen and converted to amine.
Because nitrogen does not contain many hydrogen atoms, it was thought that the production of secondary amines & high amines would result in an extremely unstable product.
The alkyl isocyanides, that are chemicals with a disagreeable odor, are also noteworthy. As a result, we exclusively employ this test to identify basic amines.
Why are secondary amino acids far more basic as primary and secondary amino acids, and what is the carbylamine formula?
When compared to the first and second amines, there are a few reasons why the secondary amine is the most fundamental. Next, let’s talk about this.
Amines are hydrocarbon groups that substitute one, two, or all three hydrogen atoms in ammonia.
The main amine is formed when hydrogen is replaced by a single group [for example, methylamine (CH3-NH2)], the secondary amine is formed when hydrogen is replaced by two groups [for example, Dimethylamine (CH3-NH-CH3)], and the high amine is formed when hydrogen is replaced by three groups [for example, trimethylamine is CH3-N(CH3) -CH3].
Amines are important in the environment because they contain fewer electrons than nitrogen. As a result, they have a high proclivity for donating single electrons to electron receivers.
Synthesis of Isocyanide
1. From Formamides to Isocyanide Synthesis
Dehydration and isocyanides are frequently used together. Before adding the base, such as triethylamine or pyridine, formamide is dehydrated using toluene sulfonyl chloride, phosphorus oxychloride, phosgene, diphosgene, or Burgess reagent.
2. Derived from a dichlorocarbon
The soluble base reacts with chloroform to produce dichlorocarbene in the carbylamine reaction (Alkyl isocyanide test is given by Hofmann isocyanide test).
The four major four are then converted to isocyanides by carbene.
Demonstrating the formation of tert-butyl isocyanide using tert-butylamine in the context of a binding quantity of benzyl triethylammonium chloride as a catalyst transfer phase.
Conclusion
The inclusion of an amine to the intermediate formed by the dehydrohalogenation of chloroform is part of the carbylamine reaction process. Dichlorocarbene is the resultant intermediate. The dichlorocarbene intermediate is an extremely reactive intermediate.
Hofmann isocyanide synthesis is another name for the carbylamine reaction. Isocyanides cannot be made from secondary or tertiary amines using the carbylamine reaction.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



