The atomic radius of a chemical element is a measurement of the atom’s size, usually the average or typical distance between the nucleus’s core and the outermost isolated electron. There are several non-equivalent definitions of atomic radius since the border is not a well-defined material thing. Van der Waals radius, ionic radius, metallic radius, and covalent radius are four commonly used concepts of atomic radius. Usually, atomic radius is measured in a chemically linked condition due to the difficulties of isolating atoms to measure their radii independently; nevertheless, theoretical results are simpler while examining atoms in isolation.
Definition of Atomic Radii
The total length from an atom’s nucleus to the outermost orbital of electrons is known as the atomic radius. In simplest terms, it can be compared to the radius of a circle, where the nucleus is at the center and the outermost orbital of the electron is at the periphery. As you walk up and down the periodic table, you’ll notice patterns that help describe how atomic radii fluctuate.
The net positive charge felt by the valence electron is the effective nuclear charge (Zeff) of an atom. Because the core electrons hide some positive charge, the valence electron does not feel the complete positive charge. Here is a full explanation of shielding and effective nuclear charge. The atomic size of an atom is substantially influenced by Zeff . As the Zeff lowers, the atomic radius increases because the electrons are screened away from the nucleus more, lowering the interaction between both the nucleus as well as the electron. Because Zeff reduces as you move down a group & right to left along the periodic table, the atomic radius increases as you move down a group and right to left.
Varieties of Radius in Relation to Bond Types
The atomic radii are difficult to calculate because the location of the outermost electron is unclear — we don’t totally know where the electron is. The Heisenberg Uncertainty Principle can explain this behavior. We determine the radius depending on the distance between both the nuclei of two bound atoms to acquire a precise, but still imperfect, estimate of the radius. Atoms’ radii are thus controlled by the bonds that they make. An atom’s radius differs based on the bond it makes, hence there is no such thing as an atom’s fixed radius.
Covalent Radius
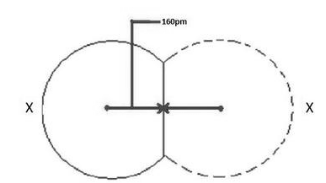
The covalent radius can be calculated when two atoms have formed a covalent connection. When atoms or ions of the same element are covalently connected, the radius from each atom is half the length between the nuclei since the electrons are drawn in opposite directions. The diameter of an atom is determined by the distance between two nuclei, because you want the radius, which really is half the diameter.
Ionic Radius
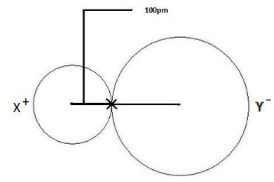
In an ionic link, each atom has a different radius than in a covalent bond. The fact that the atoms in an ionic bond are of vastly different sizes accounts for the variation in radius. One of the atoms is a cation, which is smaller than the other, and the other is an anion, which is much larger. To account for this disparity, one must first calculate the entire distance between any two nuclei and divide it by the atomic size. The larger the atomic size, the greater the radius.
Metallic Radius
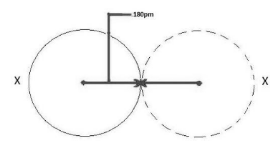
The radius of an atom linked by a metallic connection is known as the metallic radius. The distance between each atom in a metal will be the same because it is made up of atoms of different elements.
Periodic Trends of Atomic Radius
- As that number of electronic shells on an atom grows, the radius of the atom grows as well.
- When you move from the left to the right of a specific period, the size of an atom generally decreases.
Order of atomic radius
The atomic radius is the distance between the nucleus and the atom’s outermost shell. The effective nuclear charge (the net positive charge that the electrons may feel whenever the core electrons cloud the valence electrons to feel the attraction from) grows as you move from left to right in a period. This increased effective nuclear charge attracts electrons to itself, resulting in a drop in radius and so a smaller atomic radius.
The components Lithium is in the first group, Be is in the second group, but Boron is in the 13th group, and because they are in the same initial period, the atomic radius will drop as you move from left to right. As a result, the right order is Li > Be >B.
Conclusion
When attempting to describe the behaviour of atoms or compounds, the size of atoms is critical. The atomic radius is one of the ways we can express the size of atoms. This information explains why some molecules fit together and why others have portions that become too crowded under specific circumstances.
Orbital borders, on the other hand, are hazy and, in reality, vary depending on the circumstances. The distance between the nuclei of two similar atoms linked together is measured to standardise the measurement of atomic radii. One-half the distance between the nuclei of identical atoms that are linked together is known as the atomic radius.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




