Orbitals are regions of space surrounding the nucleus where electrons flow. These orbitals can be thought of as shells that surround the nucleus. The nucleus is surrounded by major energy levels called shells. Shells are further subdivided into subshells, which are energy levels of similar energy. The labels s, p,d,f and g are assigned to these subshells. Subshells are made up of orbitals, which are regions of space around the nucleus where electrons can be located, as mentioned earlier. The energy of subshells within a shell increases in the following order: s<p<d<f<g.
Electron Shells
We’ll begin by depicting the arrangement of electrons around an atom in a very basic manner. Around the nucleus of an atom, electrons are organised into energy levels, or shells. The lowest energy electrons are those in the first energy level (energy level 1), which are closest to the nucleus. Higher energy electrons will be found further away from the nucleus. The electron shell of an atom can hold 2n² electrons, with n indicating the energy level. The first shell, for example, can hold two electrons ( 2 ×1² or 2 ×2² or 8 electrons, can fit in the second shell.
Atomic Orbitals
Though electrons can be depicted as simple circles surrounding the nucleus, electrons actually follow far more intricate pathways. Atomic orbitals, or subshells, are the names for these pathways. There are several other orbital shapes s,p,d and f but for now, we’ll concentrate on s and p orbitals. One s orbital is found in the first energy level, one s orbital and three p orbitals are found in the second energy level, and one s orbital, three p orbitals, and five d orbitals are found in the third energy level. The s orbital has a lower energy than the p orbitals within each energy level.
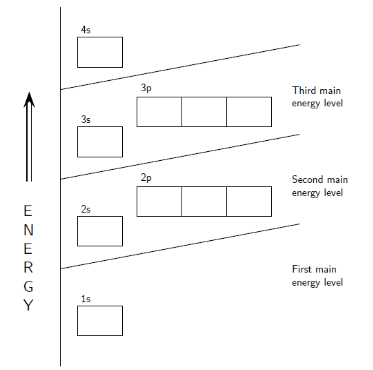
- An orbital diagram aids in determining an element’s electron arrangement. The electron configuration of an element is the arrangement of electrons in the shells. There are a few rules to follow when figuring out this configuration:
- Only two electrons can fit in each orbital. An electron pair is a group of electrons that occur together in an orbital.
- An electron will constantly strive to reach the lowest-energy orbital.
- An electron can occupy an orbital by itself, but it prefers to share a lower-energy orbital with another electron before moving to a higher-energy orbital. In other words, electrons will fill a s orbital before commencing to fill p orbitals within a single energy level.
- Two electrons can be held in the s subshell.
- Six electrons can be held in the p subshells.
In both inorganic and organic chemistry, electron configurations can be employed to rationalise chemical properties. It’s also used to decipher atomic spectra, a technique for determining the energy of light emitted by elements and compounds.
Subshells
- Within a shell, a subshell is the collection of states described by the azimuthal quantum number,l. The subshells s, p, d, fare represented by the numbers l=0,1,2,3. 2(2l+1),) is the greatest number of electrons that can occupy a subshell. This results in two electrons in the s subshell, six in the psubshell, ten in the d subshell, and fourteen in the f subshell.
- So, to summarise, All electrons in the same shell have the same value for n (the fundamental quantum number).
- All electrons with the same l(angular momentum quantum number, or orbital shape) reside in the same subshell within a shell (same n).
Periodic Table and Electron Configuration
The atomic number, or how many protons each element has, determines its position on the periodic table. Because the number of electrons in a neutral atom is equal to the number of protons, we can simply calculate electron number from the atomic number. Additionally, the column, or group, and row, or period, of an element in the periodic table provide useful information on how those electrons are grouped.
The distribution of electrons in an element’s atomic orbitals is described by its electron configuration. Atomic electron configurations are represented by a standard notation in which all electron-containing atomic subshells (with their number of electrons stated in superscript) are arranged in a sequential order. On the other hand, standard notation frequently results in extended electron configurations (especially for elements having a relatively large atomic number). In some cases, a shortened or condensed notation may be used instead of the standard notation. In the abbreviated notation, the series of completely full subshells that correspond to a noble gas’s electron configuration is replaced with the noble gas’s symbol in square brackets.
Conclusion
Orbitals are regions of space surrounding the nucleus where electrons flow. The nucleus is surrounded by major energy levels called shells. Shells are further subdivided into subshells, which are energy levels of similar energy. The labels s, p,d,f and g are assigned to these subshells. The atomic number, or how many protons each element has, determines its position on the periodic table. The s orbital has a lower energy than the p orbitals within each energy level. The distribution of electrons in an element’s atomic orbitals is described by its electron configuration. The electron shell of an atom can hold 2n2 electrons, with n indicating the energy level.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




