Faraday’s laws of electrolysis are chemistry’s quantitative laws that are used to express the magnitudes of electrolytic effects. According to the first law, the amount of chemical change caused by a current at an electrode-electrolyte interface is proportional to the amount of electricity used, whereas the second law states that the amount of chemical change caused by the same amount of electricity in different substances is proportional to their equivalent weights. In electrolytic processes, a substance’s equivalent weight is the gramme formula weight associated with a unit gain or loss of electron. The amount of electricity required to produce a chemical change in one equivalent weight unit is measured in Faradays (F). It is equal to 9.6485309 × 104 coulombs in electrical terms .
Faraday’s Law of Electrolysis
According to Faraday’s law of electrolysis, the amount of substance produced at each electrode is directly proportional to the amount of charge passing through the cell. Of course, this is a simplified version of the situation. The molar amounts of substances with different electron/atom or ion oxidation/reduction changes will not be the same. However, when those additional ratios are considered, the law is always valid.
Faraday’s Second Law of Electrolysis
When the same amount of power is transmitted through various electrolytes, Faraday’s second law of electrolysis asserts that the mass of the substances deposited is proportionate to their respective chemical equivalent or equivalent weight. As a result, “the mass of an essence deposited or enlightened at any electrode after passing a given amount of charge is directly compared to its chemical equal weight,” .
Explaination
Two cells are linked together in a series. Different electrolytes are present, and the same amount of power is carried through each item. To put it another way, the number of electron moles travelled by two electrolytes is the same.
Moles of A produced in one cell = moles of electrons actually passed mole ratio of A half reaction
Mole of B produced in other cell = moles of electrons actually passed mole ratio of B half reaction
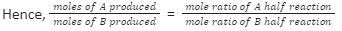
Faraday’s Second law proof:
A battery, a rheostat, and an ammeter are linked in series with two electrolytic cells having different electrolytes, CuSO4 solution, and AgNO3 solution. CuSO4 is used for copper electrodes, whereas AgNO3 is used for silver electrodes.
The cathodes are cleaned, dried, and weighed before being put into the cells. For a while, the current has been passed. The cathodes are then removed, cleaned, dried, and weighed. As a result, the deposited copper and silver masses are identified as m1 and m2.
It is found that
m1/m2 = E1/E2
having E1 and E2 being the chemical equivalents of copper and silver, respectively.
m α E
Hence, the second law is verified.
Applications of Faraday’s law:
Faraday’s Laws of Electrolysis, in their most basic form, can be used to describe a variety of metal polishing and related operations. Electroplating, metal corrosion, electrowinning, electrolytic metal ion removal from solution, and the creation of redox species are all examples. A worked example is used to demonstrate the use of Faraday’s Laws in each instance.
How Can Faraday’s First and Second Laws Be Combined?
Faraday’s first and second laws can be combined to provide the following mathematical relation:
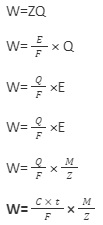
Where, Z= Electrochemical equivalent
Q= Quantity of electricity passed
E= Equivalent weight of the metal
F =1 Faraday
M = Atomic mass of the metal
C = Current past
t = Time for which current is passed
Z = Valency of the metal
Faraday ’s Constant
The Faraday constant is the quantity of electric charge carried by 1 mole of electrons, or Avogadro’s number. It is a fundamental constant in chemistry, physics, and electronics, and is usually symbolised by the italic uppercase letter F. Coulombs per mole (C/mol) is the unit of measurement.
1 Faraday = 9.65 104 C mol-1 (coulombs per mole).
Conclusion
1) Because one Faraday (96,500 coulombs) deposits one gramme equivalent of the substance, the electrochemical equivalent can be calculated from the equivalent weight i.e.,
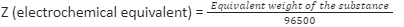
2) Knowing the weight of the substance deposited (W gramme) when a definite quantity of electricity (Q coulombs) is passed, the equivalent weight of the substance can be calculated, i.e,

 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




