Acids and bases are two classes of chemical substances that exhibit diametrically opposed characteristics and are commonly encountered in the laboratory and in everyday life. Acids, bases, and the products of their reactions are necessary for a wide variety of biological activities and are extremely important in business and agriculture.
Acids typically have a sour or tart flavour. Bases are often harsh in flavour and might feel slick to the touch. However, powerful acids and bases are toxic and can cause chemical burns; they should never be ingested or handled.
When acids and bases react with other substances, such as water, they dissociate, or break up, to form ions, charged particles. A hydrogen atom is composed of one proton with a positive charge and one electron with a negative charge (negative charge). The atom’s positive and negative charges balance out, leaving the hydrogen atom neutral—it has no net charge. When a hydrogen atom loses its electron, it transforms into a positively charged ion. Due to the fact that the hydrogen ion now contains just one proton, the words hydrogen ion and proton are occasionally used interchangeably in acid-base chemistry.
Acids and bases exhibit a variety of characteristics that aid in their identification. However, they are most precisely described by their ability to shed or acquire a hydrogen ion.
Ionization of Acids and Bases
The ionisation of a chemical refers to the process by which neutral molecules are converted into charged ions when they are exposed to a solution. Aqueous acids, according to the Arrhenius theory, are chemicals that dissociate in the presence of water in order to form hydrogen ions, H+, in the presence of aqueous solution.
Ionization of Acids
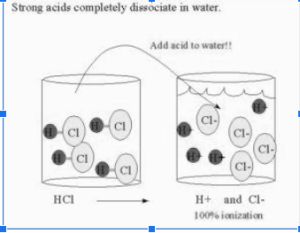
The degree of ionisation is a measure of the acidity or alkalinity of a solution. A strong acid is defined as one that totally ionises in water, whereas a weak acid is defined as one that only partially ionises in water. As there are various degrees of ionisation of acids, there are also various degrees of weakness, each of which may be expressed in a straightforward quantitative manner.
The following chemical equation and equilibrium constant expression can be used to describe the ionisation of a weak acid, because it is an equilibrium reaction.
HA (aq ) + H2O ( l ) H3O+ ( aq ) + A–
Ka = [ H3O+ ] [A–] / [HA]
The Acid Ionisation Constant is defined by the Equilibrium Constant for the ionisation of an acid (Ka). The acid ionisation constant, on the other hand, will increase in proportion to the strength of the acid (Ka). This suggests that a strong acid is a more effective proton donor in this situation. Since the concentration of the product in the numerator of the Ka increases with increasing acid strength, the larger the acid ionisation constant becomes (Ka).
Ionization of Bases
In an aqueous media, some bases, such as lithium hydroxide and sodium hydroxide, totally dissociate into their ions, and they are referred to as strong bases. As a result, the ionisation of these bases produces hydrochloric ions, denoted by the symbol (OH–). The following is a comparable formula for the bases:
A + H2O OH– + HA+
Kb = [ OH–] [ HA+] / [ A]
The base ionisation constant, abbreviated Kb, refers to the equilibrium constant for the ionisation of a base in a chemical reaction. Because of this, a strong base is indicative of a good proton acceptor, whereas an acidic base is indicative of a good proton donor. The dissociation of weak acids or weak bases in water is represented by the equation:
CH3COOH + H2O ⇔ CH3COO‾ + H3O+
NH3 + H2O ⇔ NH4+ (aq) + OH‾ (aq)
Arrhenius Theory
Because the majority of acid and base ionisation happens in an aqueous medium, the Arrhenius theory is extremely useful in describing how acids and bases ionise. We can determine the strength of acids and bases based on the degree of ionisation that occurs between them. In addition, the degree of ionisation varies for distinct acidic and basic chemicals from one another. In an aqueous solution, some acids, such as hydrochloric acid (HCl) and perchloric acid (HClO4), totally break down into their constituent ions.
All of these acids are referred to as strong acids because of their potency. Due to the formation of hydrogen ions during acidification, these chemicals serve to donate protons to the cell. The same may be said for some bases, which are examples of which are sodium hydroxide (NaOH) and lithium hydroxide (LiOH) which totally separate into their ions when placed in an aqueous solution or medium. These foundations are referred to as strong foundations. The ionisation of these bases results in the formation of hydroxyl ions (OH–).
Because of this, the degree to which acids and bases ionise relies on the degree to which compounds are dissociated into their constituent ions during the reaction. If the ionisation of strong acids and bases is compared to that of weak acids and bases, the strong acids and bases have a higher degree of ionisation. A strong acid suggests a good proton donor, whereas a strong base implies a good proton acceptor – for example, the dissociation of the weak acid HA is an example of this.
HA (aq) + H2O(l) ⇌ H3O + (aq) + A–(aq)
Arrhenius Concept of Acid and Base Ionization
Arrhenius’ theory states that an acid is a substance that breaks down in an aqueous medium, releasing hydrogen ions as a result. A base, on the other hand, is a chemical that, when present in an aqueous media, creates hydroxyl ions. The idea of Arrhenius is extremely significant in understanding the ionisation of acids and bases. This is due to the fact that ionisation happens most frequently in aqueous media. The degree to which an acid and a base are ionised can be used to determine the strength of the acid and the base, respectively.
The degree of ionisation is determined by the amount of acidic and basic chemicals present. If you put some acids in an aqueous solution, they entirely dissociate into ions. Examples include perchloric acid and hydrochloric acid. Strong bases are what these kinds of bases are referred to as. Ionization of these bases results in the formation of hydroxyl ions. Consequently, the amount of acid-base ionisation relies on the amount of dissociation that occurs at the ions of each chemical. When compared to weak acids and weak bases, strong acids and strong bases have a larger degree of ionisation.
Conclusion
The Arrhenius hypothesis states that the strength of an acid or a base is proportional to their ability to act as a source of H+ and OH- ions in an aqueous medium.
According to the Bronsted-Lowry theory, a strong acid rapidly gives a proton, and a strong base readily absorbs one.
The strength of an acid is defined as its proclivity to donate protons, whereas the strength of a base is defined as its proclivity to take protons.
The conjugate bases of strong acids are weak, whereas the conjugate bases of weak acids are strong.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




