Number of Atoms
When it comes to elements, the atomic number of an element is determined by the number of protons present in it, and it is used to distinguish one element from the next. It is established by the number of protons and neutrons coupled that an element has a specific mass number.
What is the definition of an atomic number
- The atomic number of an atom is determined by counting the total number of protons in the nucleus of that atom.
- It is denoted by the letter ‘Z,’ which stands for zero.
- All of the atoms of a specific element contain the same amount of protons, and so have the same atomic number, as well as other characteristics.
- Atoms of different elements have atomic numbers that differ from one another.
- For example, all carbon atoms have an atomic number of 6, yet all oxygen atoms have 8 protons in their nucleus, which is the same as the atomic number of carbon.
The Evolution of the Atomic Number
Atoms are now taken for granted, yet they were once regarded as unbelievably for thousands of years. The theory that the cosmos is made up of small indivisible pieces, which they termed atoms, was first proposed by a Greek philosopher named Leucippus and his pupil Demokritos around 2500 years ago. The famous Greek philosopher Aristotle, on the other hand, did not share their sentiments. Because Aristotle’s beliefs were widely accepted throughout Europe for about 2000 years, the concept of atoms was put on hold for several centuries.
Atoms are the fundamental constituents of matter. Innumerable combinations are possible, resulting in the formation of many compounds. Protons, neutrons, and electrons are found in every atom, with the exception of the common form of hydrogen. It is equal to the amount of protons in an element’s nucleus that determines the element’s atomic number. It is the energy level around the nucleus in a neutral atom that is equal to the number of protons in shells, in which case the atom is considered neutral.
Isotopes are atoms that have the same atomic number but have different neutron numbers, and so have different mass numbers from one another.. In an element in a specific environment on Earth, its standard atomic weight is determined by the average isotopic mass of an isotopic mixture of that element’s atoms. It is estimated that slightly more than three-quarters of all naturally occurring elements exist as a mixture of isotopes; the average isotopic mass of an isotopic mixture for an element in a specific environment on Earth is used to calculate the element’s standard atomic weight.
Atomic Numbers in Action: Exemplifications
When it comes to atoms, the atomic number is equal to the number of protons in the nucleus of an atom, or the number of electrons in an electrically neutral atom.
Numerical representation of atomic number: number of protons.
To give an example, there are 11 electrons and 11 protons in the nucleus of the element sodium. In this case, the atomic number of the Na atom equals the sum of the numbers of electrons and protons.
Atomic Number Orbital Energy Levels
It is more likely that an electron will be located in particular areas of a specific energy level when it is at a specific energy level than when it is at a specific energy level. Those who work in this field are known as orbitals. Oscillates with the same energy make up sublevels, which are themselves composed of orbitals. Elementary orbitals can hold a maximum of two electrons at a time.
In order to depict the arrangement of electrons in an atom, the most commonly used method is to make diagrams such as those illustrated in the diagram.
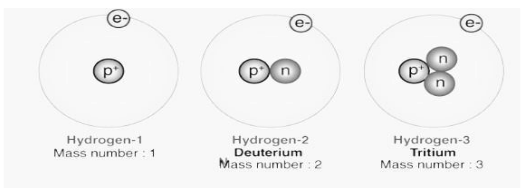
The number of electrons in each energy level must be recorded on a piece of paper. It is possible to determine the atomic number of an element by counting the number of electrons contained within its atoms. The atomic number of carbon is 6, which means that it has six electrons, which are represented by the numbers 2,4, and 5. As a result, an atom with the atomic number 12 has an electronic structure of 2, 8, 2, with two electrons in the outermost energy level, eight in the next energy level, and two at the highest energy level at the outermost end of the structure. The most straightforward way to comprehend these arrangements is to examine a large number of examples.
Periodic Table of the Modern Era
The modern periodic table, often known as the extended form of the periodic table, is based on the modern periodic law. The table provides a list of items in ascending atomic number sequence. The current periodic table is known as the modern periodic table. There are 18 vertical columns and 7 horizontal rows in all.
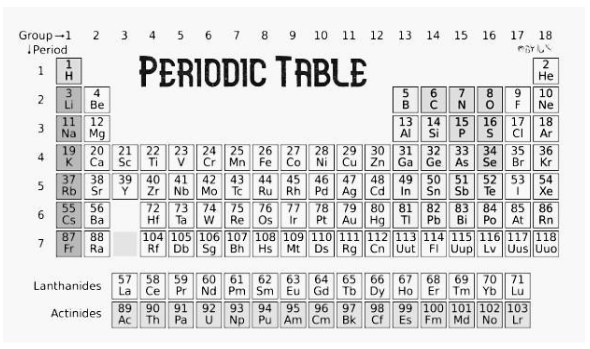
- The contemporary periodic law asserts that an element’s physical and chemical properties are periodic functions of its atomic number.
- Across each row, scientists organised elements in ascending order of their atomic numbers from left to right. And it was discovered that elements with comparable qualities repeat themselves at regular intervals.
- Atomic mass is the sum of the protons and neutrons in an atom’s nucleus. The atomic number, on the other hand, refers to the number of protons in a nucleus. In addition, the number of protons in the nucleus equals the number of electrons outside the nucleus.
- The nucleus is well-known to be buried deep within an atom. However, electrons outside of it, particularly those in the outermost shell, are free to migrate. As a result, they are involved in chemical reactions. As a result, an element’s properties are determined by the atomic number rather than the atomic mass.
Conclusion
The atomic number of an element is determined by the number of protons present in it, and it is used to distinguish one element from the next. It is established by the number of protons and neutrons coupled that an element has a specific mass number.
Atoms are the fundamental constituents of matter. Innumerable combinations are possible, resulting in the formation of many compounds. Protons, neutrons, and electrons are found in every atom, with the exception of the common form of hydrogen. It is equal to the amount of protons in an element’s nucleus that determines the element’s atomic number.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




