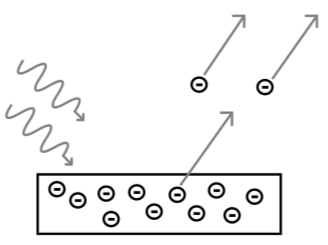
The photoelectric effect occurs when light shines on a metal and causes electrons to be expelled from the metal’s surface. Photoemission is another name for this process, and photoelectrons are the electrons that are emitted from the metal. Photoelectrons behave and behave like other electrons in terms of behaviour and attributes. The prefix photo- merely indicates that incoming light has expelled electrons from a metal surface.
Photoelectric Effect
When a substance absorbs electromagnetic radiation, electrically charged particles are emitted from or within it, causing the photoelectric effect. The ejection of electrons from a metal plate when light falls on it is a common definition of the effect. The radiant energy may be infrared, visible, or ultraviolet light, X-rays, or gamma rays; the substance could be a solid, liquid, or gas; and the released particles could be ions (electrically charged atoms or molecules) or electrons. Because of the perplexing concerns it presented about the nature of light—particle vs wavelike actions—the phenomena was vitally important in the development of modern physics, which were finally resolved by Albert Einstein in 1905. The effect is still essential in study in fields ranging from materials science to astrophysics, as well as in the development of a range of useful gadgets.
Characteristics of the Photoelectric Effect
The photoelectric effect has three key characteristics that conventional physics cannot explain:
(1) the lack of a lag period
(2) the independence of photoelectrons’ kinetic energy from input radiation intensity,
(3) the presence of a cut-off frequency. Let’s take a closer look at each of these traits.
The Absence Of Lag Time
Even at very low intensities of incident radiation, electrons are emitted almost instantly when radiation reaches the target material in the electrode. This lack of lag time runs counter to our knowledge of classical physics. According to classical physics, it would take a long time for irradiated electrons to accumulate enough energy to leave the electrode surface for low-energy radiation; yet, no such energy building has been seen.
The Independence Of Photoelectrons’ Kinetic Energy From Input Radiation Intensity
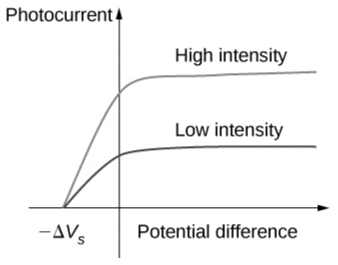
typical experimental curves in which the photocurrent is plotted against the applied voltage difference between the electrodes. The current develops steadily for the positive potential difference until it hits a plateau. Extending the potential increase past this point has no effect on the photocurrent. A higher photocurrent value is produced by a higher intensity of radiation. The value of the photocurrent drops as the absolute value of the potential difference increases for the negative potential difference, until it reaches zero at the stopping potential. The stopping potential value remains constant regardless of the intensity of incident radiation, whether it is high or low.
The Presence of A Cut-Off Frequency
Photocurrent does not exist below a certain frequency of incident radiation for any metal surface. The photoelectric effect’s cut-off frequency is determined by a physical feature of the metal: The cut-off frequency varies depending on the substance. A typical linear trend may be seen in the experimental data. With increasing frequency of incoming light, the kinetic energy of photoelectrons at the surface grows linearly. For all metal surfaces, measurements yield linear plots with a single slope. None of the reported occurrences are consistent with traditional notions of nature.
The Work Functions
Einstein explained the photoelectric phenomenon in 1905. Planck’s hypothesis regarding energy quanta, Einstein reasoned, should work to describe energy absorption from electromagnetic radiation by the surface of a photoelectrode if it was true for describing energy exchange between electromagnetic radiation and cavity walls. He proposed that the energy of an electromagnetic wave is carried in distinct packets. Because Einstein’s postulate states that light is made up of energy quanta, it goes beyond Planck’s theory. It states, in other words, that electromagnetic waves are quantized.
A beam of monochromatic light of frequency f is made of photons in Einstein’s approach. A photon is a light particle. Each photon has an energy quantum Ef and travels at the speed of light. The energy of a photon is solely determined by its frequency, f. A photon’s energy is expressed explicitly as
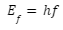
Planck’s constant is denoted by h. Photons arrive at the metal surface and each photon provides all of its energy to only one electron on the metal surface in the photoelectric effect. There are no fractional transfers in which a photon loses only part of its energy and survives, as this energy transfer from photon to electron is of the “all or nothing” type. A quantum phenomenon occurs when a photon either transfers all of its energy and ceases to exist, or when there is no transfer at all. This is in contrast to the traditional model, which allows for fractional energy transfers. With this knowledge of quantum mechanics, the energy balance for an electron on a surface receiving the energy Ef from a photon may be calculated.
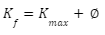
Threshold Frequency
The threshold frequency of incoming radiation is defined as the lowest frequency at which photoelectric emission or electron emission is not achievable.
The threshold frequency is the light frequency that causes an electron to dislodge and emit from the metal’s surface.
If represents incident photon frequency and the represents threshold frequency, then;
- If Y<Yth then there will be no photoelectron ejection.
- If Y=Yth photoelectrons are simply expelled off the metal’s surface, but the electron’s kinetic energy is zero.
- If the photoelectrons are discharged from the metal surface, the value is Y>Yth. The photoelectrons that are expelled have kinetic energy.
The photoelectric effect is the name given to these patterns.
As illustrated below, kinetic energy (K.E) is equal to half of the mass (abbreviated as m) multiplied by the square of the electrons’ velocity (abbreviated as v).
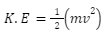
Conclusion
The page contains all of the critical information that a student needs to know about the Photoelectric Effect at a basic level, such as its Characteristics, work function and so on. This is a vital piece of equipment for taking Photoelectric Effect.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




