Hyperconjugation (π-conjugation or no-bond resonance) is a term used in organic chemistry to describe the delocalization of electrons involving mostly ∑-character bonds. Hyperconjugation usually occurs when electrons in a sigma (𝜎*) orbital (e.g. C–H or C–C) engage with a nearby unpopulated non-bonding p or antibonding p orbital. 𝜎* or π* orbitals to produce a pair of molecular orbitals that are expanded However, in what is known as negative hyperconjugation, low-lying antibonding 𝜎* orbitals may interact with filled orbitals of lone pair character (n).
Definition
It is a general stabilising interaction in which the sigma electrons of an alkyl group directly
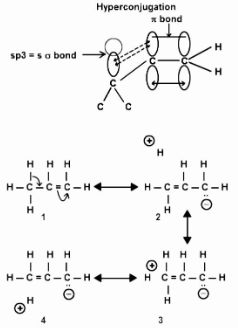
connected to an atom of an unsaturated system are delocalized. It ensures the molecule’s stability.
Types of Hyperconjugation
Such hyperconjugation is represented by contributions from structures such in the above hyperconjugation structure.
(A formal bond is indicated by the dotted line in, which indicates that electrons on the two atoms are coupled.) A structure like this is unusual in and of itself, because there is no true link connecting the hydrogen to the carbon. This is only a basic way of saying the carbon-hydrogen bond isn’t a single bond, that the C(2)-C(3) bond has some double bond character, and that the C(1)-C(2) bond has some single bond character.
The carbon-carbon “single” bond in propylene is 1.50 A for a pure single bond, which is consistent with the partial double bond nature.
The bigger the number of contributing structures such as, the greater the delocalization of electrons, and the more stable the alkene, the more alkyl groups are present in doubly linked carbon.
Because there is one less real link in structures than in the first, hyperconjugation of the type described above is known as sacrificial hyperconjugation. Isovalent hyperconjugation, on the other hand, is a type of hyperconjugation that does not require the “sacrifice” of a bond, as we saw with free radicals and carbocations.
Reverse Hyperconjugation
Reverse hyperconjugation, also known as negative hyperconjugation, is a situation in which an electron contact is directed from the pi bond to the sigma bond rather than from the sigma bond to the pi bond. To put it another way, electrons go from the pi to the sigma bond.
Hyperconjugation Applications
- Alkene stability hyperconjugation explains why some alkenes are more stable than others.
- Alkene stability is proportional to the number of alpha hydrogens in the molecule, which is proportional to resonating structures.
- The number of resonant structures and the amount of alpha hydrogens are directly proportional to the stability of alkyl carbocations.
- The size of the carbon-carbon double bond in an alkene is The single bond character will increase as the number of resonant structures increases.
- R’s electron-releasing power in alkyl benzene CH 3 is the +R group, which is utilised for electrophilic aromatic substitution due to hyperconjugation.
- Free radical stability hyperconjugation explains the stability of free radicals.
Hyperconjugation In Alkene
Delocalization of electrons, this time by overlap between a pi orbital and a sigma orbital of the alkyl group, has been attributed to the same underlying reason as stabilisation by a second double bond delocalization of electrons.
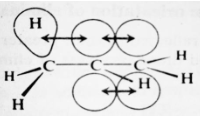
The overlap of a sigma and pi orbital in the diagram above depicts hyperconjugation in an alkene.
Individual electrons can assist bind four nuclei together to some extent because of this overlap. We call this type of delocalization, which involves a sigma bond orbital, hyperconjugation.
Trends in Hyperconjugation
Fernández and Frenking (2006) used energy decomposition analysis (EDA) to summarise the patterns in hyperconjugation among distinct groupings of acyclic compounds in a recent paper. This type of study is defined by Fernández and Frenking as “…a approach for predicting pi interactions that relies solely on the pi orbitals of the interacting pieces in “The form of the molecule.” In this type of research, the formation of bonds between different molecular moieties is a combination of three concepts. Eelstat describes a molecule’s “quasiclassical electrostatic attractions,” as described by Fernández and Frenking. The molecule’s Pauli repulsion is represented by the second term, EPauli. The third factor, Eorb, represents orbital stabilising interactions and is defined as the sum of Epi and Esigma. The sum of the three variables yields the total energy of interaction, Eint.
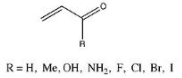
A set of enones with different substituents has their Epi values comprehensively investigated.
The methyl, hydroxyl, and amino substituents all resulted in a decrease in Epi as compared to the original 2-propenal, according to Fernández and Frenking. In contrast, halide substituents with higher atomic masses resulted in higher Epi. Because both the enone study and the Hammett analysis look at substituent effects (albeit in different species), Fernández and Frenking thought that comparing the two to look for trends could help them better understand their own findings. They discovered that the Epi values for substituted enones and the related Hammett constants have a linear connection. The graph’s slope was found to be -51.67, with a -0.97 correlation coefficient and a 0.54 standard deviation.
Methyl
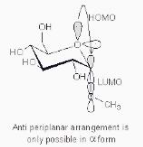
The non-bonding HOMO with a pair of electrons on the ring oxygen in -methyl glucoside is antiperiplanar to the antibonding LUMO of the C-O bond in the methoxy group. This facilitates
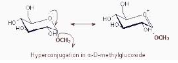
hyperconjugation between them, stabilising the -form in the process.
The methoxy group in -methyl glucoside, on the other hand, is in an equatorial position and cannot participate in hyperconjugation because it is not antiperiplanar to the lone pair on the ring oxygen. As a result, -methyl glucoside has a lower stability than -methyl glucoside.
Conclusion
Finally, delocalization of electron density is referred to by three terms: conjugation, hyperconjugation, and resonance (Be always careful to say that electron density is delocalized, not electrons). A better phrase may be electron dispersion. The distribution of electrons can be quite varied, resulting in significant changes in stability that are critical for understanding organic chemistry. Because, whatever else is true, the necessary orbitals are appropriately aligned and overlapped, appropriate concepts should be provided in a logical order as they relate to conjugation.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




