When two or more compounds have the same molecular formula but distinct structural formulas, this is referred to as structural isomerism.
They’re best appreciated in terms of their visual or fully shown structural formula
When isomers are compared, each molecule has at least one atom linked to a distinct atom.
The structural isomerism sub-divisions (a) through (d) are discussed below.
a.Chain isomerism
b.Tautomerism
c.Positional isomerism
d.Functional group isomerism
Positional isomers of C2H3X3 and C2H4X2 where X = halogen
Physical parameters, such as boiling temperatures and liquid densities, differ in all of these circumstances.
The molecular formula C2H4X2 will give rise to two positional isomers i.e. 1,1-di … and 1,2-di …
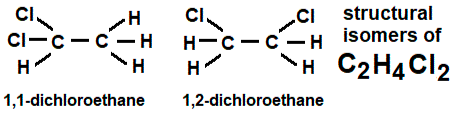
1,1-dichloroethane and 1,2-dichloroethane
57.3 and 83.7oC boiling temperatures, 1.178 and 1.253 g/cm3 densities
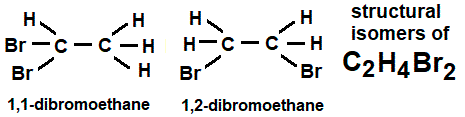
1,1-dibromoethane and 1,2-dibromoethane are two types of dibromoethane.
Boiling points are 110°C and 132°C, respectively, and densities are 2.055 and 2.180 g/cm3.
The chemical formula C2H3X3 yields two positional structural isomers,
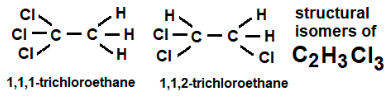
1,1,1-trichloroethane and 1,1,2-trichloroethane, respectively, where X = chlorine Cl.
Boiling points: 74 and ~112oC, densities: 1.320 and 1.435 g/cm3
Positional Isomers of Molecular Formula C3H7Br
Substituent groups, such as halogen or amino groups, can take up different locations on the carbon chain after an alkane has at least three carbon atoms.
The free radical substitution reaction can create two initial mono-substitution products in the ultraviolet light catalysed reaction of bromine and propane gases.
CH3CH2CH3 + Br2 → {CH3CH2CH2Br or CH3CHBrCH3} + HBr
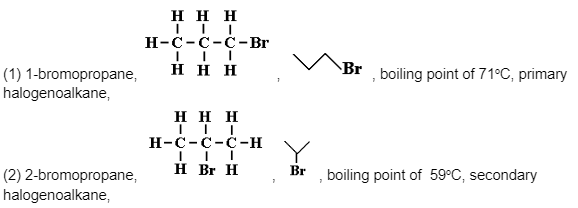
Only two isomers are possible.They’re both colourless, low-boiling liquids, but the more compact molecule (2) has a lower boiling point – they’re physically comparable, but their boiling points are different.
Chemically they are very similar e.g. both undergoing all the nucleophilic substitution reaction with ammonia, cyanide ion, and hydroxide ion etc.
In the case of the latter, (1) would result in the main alcohol, propan-1-ol, while (2) would result in the secondary alcohol, propan-2-ol – two quite distinct, if related, products from the same reaction.
For higher bromoalkanes e.g. 1-bromobutane CH3CH2CH2CH2Br and 2-bromobutane CH3CH2CHBrCH3, another chemical difference will show up on refluxing them with ethanolic potassium hydroxide, by which, following an elimination reaction,
But-1-ene CH3CH2CH=CH2 is the lone isomeric elimination product of 1-bromobutane, whereas 2-bromobutane can produce two isomeric elimination products. namely
but-1-ene CH3CH2CH=CH2, and but-2-ene CH3CH=CHCH3, i.e. you can eliminate either side of the C-Br bond.
Other halogenoalkane positional isomers …

Even with a lesser alkane like butane, there are multiple positional isomers with several distinct substituents.
Two Linear Positional Isomers of Molecular Formula C5H10
Two structural isomers result from the various positions of the C=C alkene functional group in this example.
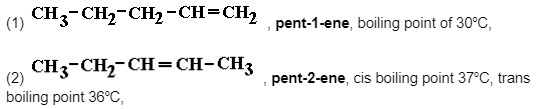
Physically, they are quite similar, for example, they are both non-polar volatile colourless liquids with low boiling temperatures. Chemically identical, for example, all of the standard electrophilic addition reactions of any alkene, however there may or may not be some ‘isomeric repercussions’ in terms of both their formation and addition reaction products, as shown in the instances below.
Pent-2-ene, unlike pent-1-ene, may be found as E/Z (cis/trans isomers).
Both can be made by cracking pentane or higher alkanes, resulting in different C5H10 isomers. Elimination reactions can be used to make them in the lab. e.g.
(a) the ‘dehydration’ of isomeric pentanols with conc. sulfuric acid or
(i) CH3CH2CH2CH2CH2OH → CH3CH2CH2CH=CH2 + H2O
Pentan-1-ol (above) can only give 1 isomer, pent-1-ene,
(ii) CH3CH2CH2CHOHCH3 → {CH3CH2CH2CH=CH2 or CH3CH2CH=CHCH3} + H2O
but pentan-2-ol (below) can give 2 isomers, pent-1-ene and pent-2-ene, because elimination of a -H (as well as the -OH) can take place either side of the >CH-OH group from an adjacent C-H.
This is not possible with pentan-1-ol with the -OH group on the end carbon.
(b) or using ethanolic potassium hydroxide in a reflux The location of the -OH in alcohols or the -Br in bromoalkanes determines the development of several isomers of the pentenes. e.g.
(i) CH3CH2CH2CH2CH2Br + KOH → CH3CH2CH2CH=CH2 + H2O + KBr
1-bromopentane can only give 1 isomer, pent-1-ene (above), but 2-bromopentane (below)
(ii) CH3CH2CH2CHBrCH3 + KOH → {CH3CH2CH2CH=CH2 or CH3CH2CH=CHCH3} + H2O + KBr
can give two isomers, pent-1-ene and pent-2-ene, because elimination of a -H (as well as the -Br) can take place either side of the >CH-Br group from an adjacent C-H.
This is not possible with 1-bromopentane with the -Br group on the end carbon.
We may also get methyl butenes (chain/positional isomers with regard to pentenes), methylcyclobutane, and dimethyl cyclopropane (both chain/functional group isomers with respect to pentenes) from the chemical formula C5H10.
Other alkene positional isomers e.g.
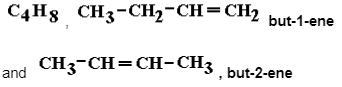
The Positional Isomers of Alcohols Based on C4H10O or C4H9OH
The molecular formula C4H10O can result in a variety of isomers, including distinct ‘types’ or ‘classes’ of alcohols based on the formula C4H9OH, each with its own set of physical and chemical characteristics, as shown below.
For the linear arrangement of the carbon chain, we have two positional isomers.
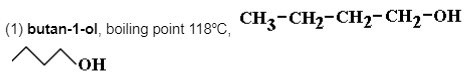
is a primary alcohol and oxidised to an aldehyde (butanal) using aqueous sulfuric acid/potassium dichromate(VI). Its fully linear structure gives it the maximum intermolecular attractive force, hence the highest boiling point.
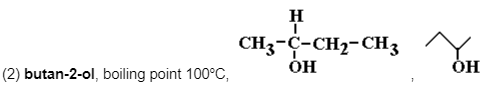
is a secondary alcohol and oxidised to a ketone (butanone) using aqueous sulfuric acid/potassium dichromate(VI).
You have two more positional isomers for the branched configuration of the carbon chain.
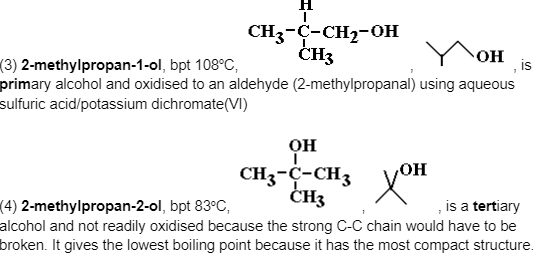
Again, note the small physical difference in boiling point, but a significant chemical difference in relative ease of oxidation.
From the molecular formula C4H10O you can also derive three ethers, (5) ethoxyethane, (6) 1-methoxypropane and (7) 2-methoxypropane.
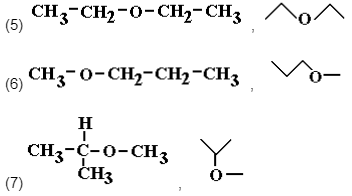
These structural isomers are derived from either changing the position of the ether linkage or configuration of the carbon chain.
Conclusion
Isomers with the same functional groups but at various places on the same carbon chain form positional isomerism, also known as position isomerism.
The chemical with the molecular formula C6H4Br2, which has three isomers: 1,2-dibromobenzene, 1,3-dibromobenzene, and 1,4-dibromobenzene, is a good example. The location of the bromine atoms on the cyclic structure differs between these isomers.
Dibromobenzene is an example of a position isomer.
Another example is the chemical C3H8O, which has two isomers: 1-propanol, also known as n-propyl alcohol, and 2-propanol, also known as isopropyl alcohol. The location of the hydroxyl group on the carbon chain differs between these isomers.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




