The term “polar bond” is used to refer to a subset of covalent bonds. We can also say that it marks the boundary between covalent bonds and ionic bonds. A polar covalent bond, on the other hand, is one between two atoms in which the electrons in each atom are unevenly distributed, thus forming the bond. Molecular dipole moments result from this state, with the two ends slightly positive or negative.
Different types of covalent bonds are determined by their electronegativity. It’s the tendency of an atom to draw electrons from other atoms that share a pair of electrons toward itself. Simply put, it’s a tendency. A polar covalent bond is the name given to the covalent bond formed between two atoms in a molecule with an electronegativity difference.
Polar Covalent Bond: An Explanation
Nonmetal atoms with different electronegativities typically form polar covalent bonds.
If there is a covalent bond between points A and B, then their electronegativity difference is greater than zero. In the bond between A and B, the shared pair of electrons move toward the more electronegative B.
There are now two charges (Poles) formed (Poles are formed and it is known as Dipolar molecular or dipole or polar covalent module) such as in H – Cl. The shared electron pair in this molecule moves toward the chlorine atom, which has a high electronegative potential. As a result, a dipole is formed, with the H atom having a partial positive charge and the Cl atom having a partial negative charge.
Polar Covalent Compounds: Properties and Applications
Stronger interactions allow for a solid state to exist for these compounds.
Unlike non-polar compounds, polar compounds have a higher melting and boiling point.
The ion mobility is responsible for their conductivity in the solution state.
Polar solvents, like water, can easily dissolve these.
Dipole Moment
A physical measure called the Dipole moment (μ) can be used to describe the polarity of a covalent bond. The dipole moment is defined as the product of the charge and the separation distance between the charges. The dipole moment is indicated by the symbol ‘μ’ and is measured in Debye (or) esu cm.
μ = e × d esu cm
d = distance between the lengths of a charge or a bond
e is an abbreviation for electronic charge.
The dipole moment of a net bond is
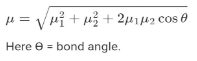
Dipole Moment Characteristics
- The dipole moment is quantifiable as a vector quantity.
- The dipole moment of non-polar molecules is zero.
- For symmetrically applicable molecules, the molecular dipole moment is equal to zero.
For example, because carbon dioxide is linear, the net bond moment is equal to zero, as the individual bond moments cancel out.
For instance, carbon tetrachloride has no dipole moment due to its very symmetrical tetrahydro structure.
- The dipole moment is used to determine a covalent bond’s percentage ionic character.
Classes of Covalent Bonds
Single Bonds
When two atoms share only one pair of electrons, a single bond is formed. It is indicated by a solitary dash (-). Despite the fact that this type of covalent bond has a lower density and is weaker than a double or triple bond, it is the most stable.
A HCl molecule, for example, contains one Hydrogen atom with one valence electron and one Chlorine atom with seven valence electrons. In this case, hydrogen and chlorine share an electron to form a single bond.
Double Binds
A double bond is formed when two participating atoms share two pairs of electrons. Two dashes (=) denote this. While double covalent bonds are significantly stronger than single covalent bonds, they are also significantly less stable.
In a carbon dioxide molecule, for example, one carbon atom has six valence electrons and two oxygen atoms have four. To complete its octet, carbon shares two of its valence electrons with one oxygen atom and two with another oxygen atom. CO2 contains two double bonds because each oxygen atom shares two electrons with carbon.
Each oxygen atom in the oxygen molecule has six electrons in its valence shell. Each atom requires two additional electrons to complete their octet. As a result, the oxygen molecule is formed when the atoms share two electrons. Due to the sharing of two electron pairs, the two oxygen atoms form a double bond.
Triple Bonds
A triple bond is formed when two atoms share three pairs of electrons. Triple covalent bonds, denoted by three dashes, are the least stable type of covalent bond.
When a nitrogen molecule is formed, for example, each nitrogen atom contributes three electrons to form three electron pairs for sharing. As a result, the two nitrogen atoms form a triple bond.
Covalent Polar Bond
When the electronegativity of the combining atoms differs, this type of covalent bond forms, resulting in unequal electron sharing. Electrons will gravitate toward atoms with a greater electronegative charge. The electronegative difference between the atoms is greater than zero but less than two.
As a result, the electron pair shared with that atom will be closer to it. Consider the formation of hydrogen bonds by molecules as a result of an unbalanced electrostatic potential. In this case, the hydrogen atom interacts with the electronegative fluorine, hydrogen, or oxygen.
Covalent Nonpolar Bond
When two atoms share an equal number of electrons, this type of covalent bond is formed. Between two atoms, the difference in electronegativity is zero.
It occurs when two atoms combine with similar electron affinities. Nonpolar Covalent Bonds are found, for example, in gas molecules such as hydrogen gas and nitrogen gas.
Elements That Form Polar Covalent Bond
Polar covalent bonds form when the electronegativity difference between two nonmetal atoms is large enough. Because the electronegativity values differ slightly, the bonding electron pair is not evenly shared between the atoms. For instance, polar covalent bonds are typically formed between hydrogen and any other non-metallic atom. Due to the large difference in electronegativity values between metals and nonmetals, they form ionic bonds with one another.
Conclusion
The term “polar bond” is used to refer to a subset of covalent bonds. We can also say that it marks the boundary between covalent bonds and ionic bonds.Stronger interactions allow for a solid state to exist for these compounds.
Unlike non-polar compounds, polar compounds have a higher melting and boiling point.
The ion mobility is responsible for their conductivity in the solution state.
Polar solvents, like water, can easily dissolve these.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




