In the discipline of organic chemistry, functional groups are substituent atoms or groups of atoms that are connected to certain molecules. These moieties (parts of the molecule found in many other compounds) are responsible for the chemical reactions that the molecule to which they are connected participates in.
In organic chemistry, a functional group is a group of atoms or bonds within a material that is responsible for the substance’s distinctive chemical reactions. The same functional group will behave similarly and experience equivalent reactions regardless of the chemical in which it is contained.
What exactly are Functional Groups?
Functional Groups are defined as a “specific grouping of components for which the distinct chemical processes of these molecules are responsible.”
Two molecules with differing sizes but the same functional groups will participate in chemical processes that are comparable or identical. The existence of a functional group in a molecule suggests that the molecule’s behavior and chemical reactions may be predicted in a systematic manner.
Understanding the features of various functional groups allows you to construct a chemical synthesis process in which chemical processes are purposely carried out in order to obtain a specified product.
Organic Chemistry Functional Groups
The way functional groups participate in a chemical process can be further modified with the assistance of other functional groups, and these groups can also be interconverted.
A few carbon-based functional groupings are depicted below.
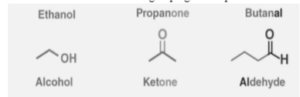
Groups of Function
As a result, functional groups are moieties that have their own specific traits and properties that are independent of the molecule to which they are linked.
The atoms of these groups, as well as the group as a whole, are joined to the molecule through covalent bonding.
In the case of polymers, functional groups are typically bonded to the nonpolar core of the carbon atoms in each repeating unit of the related polymer, infusing the carbon chain with specific chemical properties.
As shown in carboxylate salts containing the -COO– ionic group, several functional groups carry an ionic charge.
When these groups bind to molecules, they transform them into either complexions or polyatomic ions.
A ligand is the functional group that is attached to the central atom in a coordination complex.
Below are some more functional groups comprising components such as nitrogen and oxygen, with differing hybridizations of the carbon-nitrogen and carbon-oxygen bonds.
Examples of Functional Group
The presence of functional groups in a molecule impacts its solubility as well as its proclivity to form complexes. Solubility rises when the functional groups of the solute and the solvent interact well. Sugar, for example, may be easily dissolved in water since both contain the -OH (hydroxyl) group.
When a highly electronegative functional group is connected to a less electronegative atom or molecule, polarity is formed, allowing the initially nonpolar molecule to be soluble in water or other aquatic environments.
Common Functional Group Nomenclature
This subsection contains the common functional groupings, as well as the prefix and suffix that must be used in their naming. A brief summary of the composition of each of these groups is also provided.
- Hydrocarbons
The sign R represents alkanes, alkenes, alkynes (and sometimes benzene derivatives). Because they exclusively contain carbon and hydrogen atoms, these groups are sometimes known as hydrocarbyl groups. They may differ, however, in the types of bonds formed between two carbon atoms, such as double or triple bonds.
Because of the structure of the carbon-carbon bond, the reactivity of these groups varies. Some groups are made up of a lengthy, branching alkane or a ring-structured alkane that is given a name. Names like bornyl and cyclohexyl are examples.
The hydrocarbon functional groups may be ionic in nature. Carbocations are positively charged hydrocarbon structures, whereas carbanions are negatively charged hydrocarbons.
- Haloalkanes
Haloalkanes, also known as alkyl halides, are functional groups that contain a carbon-halogen link. The prefix ‘halo-‘ is used to denote a halogen. For example, the chemical CH3F can be referred to as fluoromethane, with fluoro as the prefix.
The halide suffix is used to denote a halogen. For example, fluoromethane (CH3F) can alternatively be referred to as methyl fluoride, with the suffix fluoride.
The strength and durability of the carbon-halogen bond vary depending on the halogen. The carbon-iodine link in alkyl iodides, for example, is rather weak, but the carbon-fluorine bond in alkyl fluorides is quite strong and permanent.
With the exception of these alkyl fluorides, all alkyl halides readily undergo elimination or nucleophilic substitution reactions.
- Functional Groups Containing Oxygen
The hybridization of the carbon-oxygen link completely determines the characteristics of functional groups containing a carbon-oxygen bond.
This can be explained by the electron donating impact of sp3 hybridized oxygen exhibited in alcohols, as opposed to the electron withdrawing action of sp2 hybridized oxygen observed in carbonyl groups containing a carbon-oxygen double bond.
The suffixes used in the nomenclature of compounds with a functional group that contains a C-O bond are listed below, along with examples.
It should be noted that the following table only includes a few common functional groups that contain the carbon-oxygen link. Many unusual groups with complicated compositions, such as acetal groups (RCH(OR’)(OR”) or ketal groups RC(OR’)(OR”)R”. Functional Groups Containing Nitrogen. Nitrogen-containing substituent groups may also contain carbon-oxygen linkages.
Conclusion
Functional groups are significant in chemistry because they are the component of a molecule that can undergo specific reactions. As a result, they determine the characteristics and chemistry of many organic molecules.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




