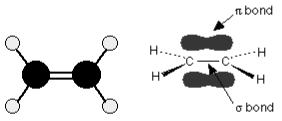
Many reactions involving alkenes, which contain carbon-carbon double bonds, include electrophilic addition. Two pairs of shared electrons form the double bond between the carbon atoms. What the diagram fails to convey is that the two pairs are not identical. One pair of electrons is retained on the line connecting the two carbon nuclei, as expected, whereas the other is held in a molecular orbital above and below the molecule’s plane.
Ethene
Electrophiles
A molecular orbital is a region of space within a molecule where a specific pair of electrons has a high likelihood of being found.
Electrophiles are molecules or ions that are drawn to electron-rich areas in other molecules or ions. An electrophile must be something that carries a full positive charge or has a minor positive charge on it somewhere because it is drawn to a negative region.
Electrophiles attack the alkenes ethene and others. In most cases, the electrophile is the slightly positive (+) end of a molecule, such as hydrogen bromide (HBr). Electrophiles are highly attracted to the exposed electrons in the pi link, and reactions occur as a result of this initial attraction.Why don’t fully positive ions like sodium, Na+, react with ethene? Although these ions may be attracted to the pi link, the process will not continue to generate bonds between sodium and carbon since sodium forms ionic bonds while carbon generally forms covalent bonds.
Covalent Bonds
When electrons from both participating atoms are shared evenly, a covalent connection is produced. A shared pair or bonding pair is the pair of electrons participating in this sort of bonding. Covalent bonds are also known as molecular bonds. Similar to noble gas atoms, the sharing of bonding pairs will ensure that the atoms reach stability in their outer shell.
Elements with extremely high ionisation energies can’t transmit electrons, and elements with extremely low electron affinity can’t absorb them. Such elements’ atoms tend to share electrons with atoms of other elements or atoms of the same element, resulting in both atoms achieving octet configuration in their respective valence shells and therefore achieving stability.A covalent bond is a relationship created by the exchanging of electron pairs between different or similar types of molecules.
Properties Of Covalent Bonds
If sharing a single electron pair between atoms is insufficient to meet an atom’s typical valence, the atoms can share several electron pairs. The features of covalent bonding are as follows:
- Covalent bonding does not result in the production of additional electrons. The bond is merely a link between them.
- They are atom-to-atom chemical connections that are extraordinarily strong.
- After they are created, covalent bonds seldom break on their own.
- The atoms that are joined have distinct orientations relative to one another, hence covalent bonds are directional.
- The melting and boiling points of most covalently bonded substances are low.
- The enthalpies of vaporisation and fusion are frequently lower in compounds containing covalent links.
- Because there are no free electrons in covalently bonded compounds, they do not conduct electricity.
- Water does not dissolve covalent compounds.
Addition Reactions
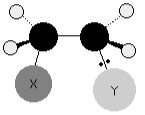
In some ways, the pi bond is superfluous. A single bond, rather than a double bond, would hold the structure together perfectly well. The electrons in the pi bond are frequently employed to attach other atoms (or groups of atoms) onto the ethene molecule. To put it another way, ethene proceeds through addition reactions. For instance, consider the molecule X-Y.
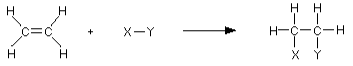
Difference Between Electrophilic and Nucleophilic Addition
The main distinction between nucleophilic and electrophilic addition reactions is that nucleophilic addition reactions involve an electron-rich component, whereas electrophilic addition reactions involve either an electron-deficient species or a neutral compound with empty orbitals.
An electron-rich chemical species that can give an electron pair to an electron-deficient species is known as a nucleophile. Electrophiles, on the other hand, are either positively or neutrally charged. It should have unoccupied orbitals to accept electrons from another species if it is neutral.
Electrophilic Addition Reaction Mechanism
The mechanism of ethene’s reaction with the molecule X-Y.
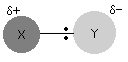
Any two separate atoms linked together are exceedingly unlikely to have the same electronegativity. We’ll assume that Y is more electronegative than X, which means that the pair of electrons will be drawn somewhat towards the Y end of the bond. This indicates that the X atom has a small positive charge.
The electrophile X atom is drawn to the exposed pi bond in the ethene. Consider what occurs as they get closer to one other.
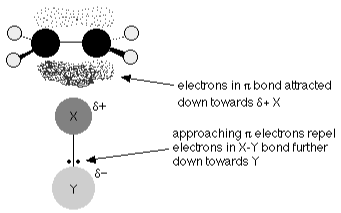
The electrons in the portion of the pi bond closest to the XY are now significantly more likely to be found. The two electrons in the pi bond travel closer to the X as the process progresses, eventually forming a covalent link. The X-Y bond’s electrons are forced totally onto the Y, resulting in a negative Y- ion.
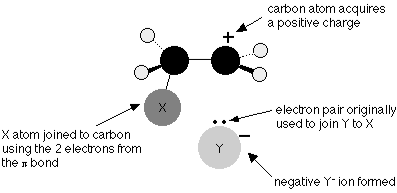
The electrons in the lone pair on the Y- ion are strongly attracted to the positive carbon atom in the final step of the reaction. They approach it and create a covalent (dative) link between the Y and the carbon.
Conclusion
A reaction in which two molecules combine to form a larger one is known as an addition reaction. In the process, nothing is lost. The larger molecule contains all of the atoms from the smaller one.An electrophilic addition reaction occurs when an electrophile attacks what we consider to be the “important” molecule. The “important” molecule has a high-electrons-density area that is attacked by something with a positive charge.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




