Hyperconjugation is a stabilising interaction that occurs when electrons in a σ-bond (typically C-H or C-C) engage with a neighbouring vacant or partially filled p-orbital or a π-orbital, resulting in an expanded molecular orbital that boosts the system’s stability.
Definition
Hyperconjugation is the interaction of σ-bonds (e.g. C-H, C-C, etc.) with a network in the formalism that splits bonds σ into π and types.
Contributing structures, such as those for toluene (below), are commonly used to illustrate this interaction. Contributing structures are sometimes called “heterovalent” or “sacrificial hyperconjugation” because they contain one two-electron bond less than the normal Lewis formula for toluene.
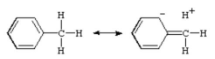
In neutral hydrocarbons, there is yet no evidence of sacrificial hyperconjugation.
Hyperconjugation is also used to describe the interaction between σ -bonds and an empty or partially filled π or p-orbital in carbenium ions and radicals. The following is an example of a contributing

structure for the tert-butyl cation:
This latter example is referred to as a “isovalent hyperconjugation” example (the contributing structure containing the same number of two-electron bonds as the normal Lewis formula).
“Double bond-no-bond resonance” can be found in both structures.
Negative hyperconjugation refers to the interaction between filled π or p orbitals and adjacent antibonding σ* orbitals, as seen in the fluoroethyl anion:

Chemical Properties of Hyperconjugation
Hyperconjugation (-conjugation or no-bond resonance) is the delocalization of electrons with the presence of mostly -character bonds in organic chemistry.
Dipole moments
A dipole moment occurs when there is a charge separation in a system. As a result, they can be found in both ionic and covalent bonds. The difference in electronegativity between two chemically linked atoms causes dipole moments.
The bond dipole moment is used to determine the polarity of a chemical bond between two atoms in a molecule. It uses the idea of electric dipole moment, which is a measure of how far negative and positive charges are separated in a system.
The bond dipole moment is a vector quantity since it has both magnitude and direction. Below is an illustration of the dipole moment that occurs in an HCl (hydrochloric acid) molecule.
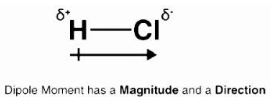
The symbols 𝛿⁺ and 𝛿⁻ denote the two electric charges that arise in a molecule, which are of equal magnitude but opposite signs. They are separated by a predetermined distance, denoted by the letter ‘d.’
Dipole Moment Formula
The dipole moment is the product of the charge’s magnitude and the distance between the positive and negative charge’s centres. The Greek letter ‘µ’ stands for it.
Mathematically,
Dipole Moment (µ) = Charge (Q) * distance of separation( r)
It is measured in Debye units, which are indicated by the letter ‘D.’ 1 D = 3.33564 × 10-30 C.m, where C stands for Coulomb and m stands for metre.
The bond dipole moment can be described as follows in a chemical bond between two atoms with different electronegativities:
μ = 𝛿.d
where the bond dipole moment is μ
𝛿 is the difference in magnitude between the partial charges 𝛿⁺ and 𝛿⁻,
The distance between 𝛿⁺ and 𝛿⁻ is denoted by d.
The bond dipole moment (μ) is a vector quantity whose direction is the same as that of the bond axis. In chemistry, the arrows that denote dipole moments start at the positive charge and end at the negative charge.
When two atoms with different electronegativities collide, the electrons tend to shift away from their original places in order to approach the more electronegative atom. The bond dipole moment can be used to describe the movement of electrons.
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic molecule composed entirely of hydrogen and carbon. Hydrocarbons are an example of group 14 hydrides. Hydrocarbons are colourless and hydrophobic, with only a slight odour. It’s impossible to generalise further due to their different molecular architectures. In the oil and gas industry, the term “hydrocarbon” refers to both petroleum and natural gas, which are two naturally occurring phases of hydrocarbons that have been commoditized by the industry. The burning of fossil fuels, including fuel production and combustion, accounts for the majority of anthropogenic greenhouse gas emissions. The emissions of vegetation are a natural supply of hydrocarbons such ethylene, isoprene, and monoterpenes.
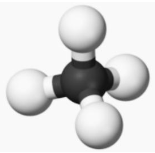
The methane molecule, CH₄, is represented by a ball-and-stick model. Methane belongs to the alkanes, a homologous group of compounds with only one bond.
Types
The categories for hydrocarbons are given by the IUPAC nomenclature of organic chemistry.
- One or more double or triple bonds exist between carbon atoms in unsaturated hydrocarbons. Alkenes are compounds with a double bond. CnH₂n is the formula for those with one double bond (assuming non-cyclic structures). Alkynes are compounds with three carbon atoms. CnH2n-2 is the formula for those with one triple bond.
- Arenes are hydrocarbons with at least one aromatic ring, also known as aromatic hydrocarbons. Aromatic hydrocarbons emitted by gasoline-powered cars account for 10% of total non methane organic carbon emissions.
- Saturated hydrocarbons are the most basic type of hydrocarbon. They are made up completely of single bonds and are hydrogen-rich. CnH₂n₊₂ is the formula for acyclic saturated hydrocarbons (alkanes). CnH₂n₊₂(1-r), where r is the number of rings, is the most general type of saturated hydrocarbons. Cycloalkanes are compounds having exactly one ring. Petroleum fuels are made up of saturated hydrocarbons, which can be found in either linear or branched forms.The property of substitution reaction is one of their distinguishing features (like chlorination reaction to form chloroform). Structural isomers are hydrocarbons with the same molecular formula but distinct structural formulas. Branched hydrocarbons can be chiral, as shown by the example of 3-methylhexane and its higher homologues. The side chains of biomolecules like chlorophyll and tocopherol are made up of chiral saturated hydrocarbons.
- Hydrocarbons with a ring structure are known as alicyclic hydrocarbons. The carbon atoms can be hybridised as Sp, Sp², or Sp³.
Uses of Hydrocarbons
- Fuels made of hydrocarbons are commonly used. LPG (liquefied petroleum gas) and CNG (compressed natural gas) are two examples (Liquefied natural gas).
- They’re employed in the production of polymers like polyethene and polystyrene.
- As a starting material, these organic compounds are used in the production of pharmaceuticals and colours.
- They are used as lubricants and grease.
Conclusion
Although this evidence is not clear, it can imply that a chemical shift has occurred: The odour has changed. A colour shift (for example, silver to reddish-brown when iron rusts). The generation (exothermic) or loss (endothermic) of heat is an example of a change in temperature or energy.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




