In order to become stable, different atoms must combine with one another. As a result of the creation of bonds, this combination happens. Ionic or electrovalent bond; covalent bond; and coordinate bond are the three types of bonds that can be formed. This, in turn, demonstrates that every relationship has some characteristic that distinguishes it from others.
The following are some of the several qualities or aspects of bonds that can be referred to as bond parameters. A particular covalent bond can be distinguished by a number of characteristics:
Bond Length, Bond Angle, Bond Strength.
Angle of Bonding
It is the angle between two bonds, or the angle between two orbitals that contain a pair of bonding electrons around the central atom, that is referred to as bond angle in a complex molecule or an ion. Bond angle is measured in degrees. This angle is typically measured in degrees, and it can be further quantified using the spectroscopic technique.
In addition to providing an accurate picture of the distribution of bound electron pairs around the atoms, this information is useful in determining how a molecule should be shaped. It also provides an indication of the distribution of bound electron pairs surrounding the atoms, which is important in identifying the structure of the molecules.
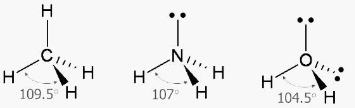
The Following Three Elements Have the Most Influence On Bond Angles
- Hybridization: The bond angle of the central atom is determined by the state of hybridization of that atom. The bond angle increases in direct proportion to the size of the s character.
sp3– 109°28′ north latitude
sp2– 120 degrees
sp- 180 degrees
- Lone pair repulsion: The presence of a lone pair of electrons at the central atom has an effect on the bond angle of the molecule. Bond angle decreases when the lone pair attempts to reject the bond pair it is trying to repel.
For example, the bond angle of CH4 (which contains no lone pair) is 109°, whereas the bond angle of NH3 (which has one lone pair) is 107°.
- Electronegativity: As the electronegativity of the central atom decreases, the bond angle of the central atom decreases.
Consider the following example:- The bond angle of NH3 is 107°, but the bond angle of PH3 is 93.5°.
The Length of The Bond
Molecular bond length is a measure of the distance between the nuclei of two chemically bound molecules in a compound or between a compound and its surroundings. It is approximately equal to the total of the covalent radii of the two atoms that are bound to one another in space. The length of covalent bonds is inversely related to the order of the bonds — greater bond orders result in stronger bonds, which are accompanied by stronger forces of attraction that hold the atoms together. These strong forces of attraction result in short bonds as a result of their strength.
In the preceding section, we showed how to express the length of a covalent bond in terms of the sum of the individual covalent radii of the atoms involved in the bond. It is possible to determine this bond parameter experimentally using one of the procedures listed below:
It is possible to use rotational spectroscopy.
X-ray diffraction is a type of diffraction.
Diffraction of neutrons.
Atoms that are bonded together have a tendency to absorb heat energy from their surroundings and to vibrate constantly. As a result of the vibration, the bond length changes. As a result, it is critical to remember that the bond length of a covalent bond is the average distance between the nuclei of the atoms that are participating in the bond.
Bond Energy (Also Known as Bond Enthalpy) Is A Type Of Energy That Can Be Transferred Between Two Objects
The bond energy of a chemical bond is a measure of the strength of the chemical connection. It can be described as the amount of energy required to break all covalent bonds of a given type present in a single mole of a chemical molecule of a specific kind (which is in its gaseous state).
Keep in mind that bond energy is not the same as bond dissociation energy, which is the opposite. It is the latter that represents the change in enthalpy associated with the homolytic cleavage of a bond, whereas it is the former that represents the average of the bond dissociation enthalpies of all bonds in a molecule (that is, of a certain type).
Factors Influencing the Bonding Energy
Chemical bonds are extremely strong, and the amount of energy required to break one is directly proportional to the strength of the bond. Thus, bond energy is defined as follows:
- Bond energies are inversely proportional to the length of the bond, meaning that longer bonds have lower bond energy.
- Bond energies are directly proportional to the order of the bonds, hence numerous bonds have high bond energies.
- The bond strength is inversely related to the atomic radii of the atoms involved in the bond (since the atomic radius is directly proportional to bond length).
Conclusion
In a complex molecule or an ion, the bond angle is the distance between two orbitals that contain a pair of bonding electrons. This angle is commonly expressed in degrees and calculated spectroscopically.
To be stable, atoms must unite. Bonds constitute this combination. The three types of bonding are ionic, covalent, and coordinate. This shows that every relationship has a unique trait that sets it apart from others.
The following are some of the bond parameters’ features or aspects. A covalent bond’s features include:
Bond Angle, Bond Strength, Bond Length, Bond Order ( which is a form of enthalpy bond).
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




