Resonance, also known as mesomerism, is a means of defining bonding in specific molecules or ions in valence bond theory by combining many contributing structures (or forms, also known as resonance structures or canonical structures) into a resonance hybrid (or hybrid structure). It’s especially useful for explaining delocalized electrons within molecules or polyatomic ions where the bonding can’t be stated by a single Lewis structure.
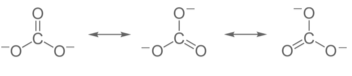
One contributing structure may have a stronger resemblance to the real molecule than another (in the sense of energy and stability). Structures with a low potential energy value are more stable and resemble the actual structure more than those with a high value. Major contributors are the most stable contributing structures. Structures that are energetically unfavorable and so less desirable are small factors. Major contributors are generally structures that are listed in rough order of decreasing importance.
- follow the octet rule as much as possible (8 valence electrons around each atom instead of deficits or surpluses, or 2 electrons for Period 1 elements).
- have a maximum number of covalently bonded atoms.
- carry a minimum number of formally charged atoms, with the separation for unlike and like charges minimized and maximized, respectively.
- place a negative charge on the most electronegative atoms and a positive charge on the most electropositive atoms.
- do not deviate significantly from idealized bond lengths and angles (e.g., the relative unimportance of Dewar-type resonance contributors for benzene).
- local aromatic sub-structures should be preserved whereas anti-aromatic substructures should be avoided.
All resonance contributors with a greater energy than the lowest-energy contributor are considered minor resonance strength. For example, for formamide, there are three probable contributors: HCONH2, HCONH3, and HCONH2.
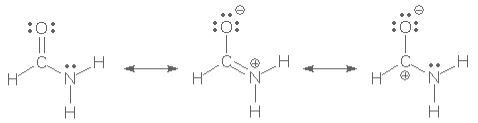
We must determine which of these forms has the lowest energy. That one will make a major contribution. All of the others will only have a minor contribution.
Some rules that help you decide, in order of importance:
- Follow the octet rule.
- Charge separation should be kept to a minimum.
- Charge the more electronegative atom with a negative charge
Octet Rule
The octet rule is a chemical rule of thumb that represents the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, resulting in an electronic configuration. The law applies particularly to elements like carbon, nitrogen, oxygen, and halogens, but also applies to metals like sodium and magnesium. Other laws, such as the duplet rule for hydrogen and helium or the 18-electron rule for transition metals, apply to other elements.
Formally charged atoms
The electric charge of an atom in a molecule is known as formal charge (FC). The number of valence electrons minus half the number of electrons shared in a bond minus the number of electrons not bound in the molecule is used to compute it. Formal charge is a method of estimating the distribution of electric charge in a molecule.
The octet rule, however, has three general exceptions: Molecules containing an odd number of electrons, such as NO; Molecules with more than eight electrons in one or more atoms, such as SF6; and. Molecules containing one or more atoms with less than eight electrons, such as BCl3.
It can be stated mathematically in the following way:
F.C. = [Total number of valence e– in free state] – [total number of e– assigned in Lewis’s structure]
F.C. = [Total number of valence e– in free state] – [total number of nonbonding pair e– (lone pair)] – 1/2 [total no. of bonding e–]
Because bonding e– is shared by two atoms, the number of bonding e– is multiplied by 1/2.
Electronegativity
The electronegativity value of the atoms involved in the Lewis structure determines how electrons travel. The rearrangement of pi and sigma bonds within a molecule is known as resonance. The molecule is technically unchanged; the identical atoms are linked together.
The tendency of an atom to attract electrons in a molecule is known as electronegativity. The complete transfer of an electron from one atom’s unfilled outer shell to the unfilled shell of another is caused by large variation in electronegativity across atoms in a given molecule.
Conclusion
By understanding the basic concept of the resonance and electronegativity of a molecule we can understand the reactive nature of any compound and the chemical bonding within the molecule.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




