In 1794, the Society of Dutch Chemists (Dutch: Gezelschap der Hollandsche Scheikundigen) was formed by physician Jan Rudolph Deiman, merchant Adriaan Paets van Troostwijk, chemist Anthoni Lauwerenburg, and botanist Nicolaas Bondt to produce 1,2-dichloroethane from olefiant gas (oil-making gas, ethylene) and chlorine gas.
1,2-dichloroethane belongs to the class of chloromethanes with two chloro groups replaced at positions 1 and 2. It functions as a non-polar solvent, a hepatotoxic agent, and a mutagen, among other things.
Properties Of 1,2-Dichloroethane
Ethylene dichloride (EDC) is a chlorinated hydrocarbon composed of the chemical composition 1,2-dichloroethane. It’s a colourless liquid that smells like chloroform. Vinyl chloride, which is used to create polyvinyl chloride (PVC) pipes, furniture and automotive upholstery, wall coverings, kitchenware, and automobile parts, is the most prevalent usage of 1,2-dichloroethane. 1,2-Dichloroethane is also utilised as a solvent and an intermediary in the production of other organic chemical compounds. Many other solvents, including water (at a boiling point of 70.5 °C or 158.9 °F or 343.6 K) and other chlorocarbons, create azeotropes with it.
Physical Properties of 1,2-Dichloroethane
- Ethylene dichloride is commonly referred to as 1,2-dichloroethane.
- Ethylene dichloride has the following chemical formula:
- The molecular weight of this compound is 98.96 g/mol.
- Ethylene dichloride is a colourless, oily, heavy liquid with a low water solubility.
- With an odour threshold of 6-10 ppm, ethylene dichloride has a nice chloroform-like odour.
- At 20 °C, the vapour pressure of ethylene dichloride is 64 mm Hg.
1,2-Dichloroethane is a common synthetic chemical in the United States that is primarily utilised as an intermediary in the synthesis of vinyl chloride.
Uses Of 1,2-Dichloroethane
Approximately 95 percent of the world’s 1,2-dichloroethane production is used in the manufacture of vinyl chloride monomers (VCM, chloroethene), with hydrogen chloride as a byproduct. Polyvinyl chloride is synthesised from VCM. 1,2-Dichloroethane was once used as a degreaser and paint remover, but it is now prohibited due to its toxicity and potential carcinogenicity. It is used as an intermediate in the production of a variety of organic compounds, including ethylenediamine, as a useful ‘building block’ reagent. In the laboratory, it is occasionally used as a source of chlorine, with the elimination of ethene and chloride.
1,2-Dichloroethane Isomerism
Isomerism isn’t usually visible at first glance. When two structures appear to be isomers, they’re actually the same chemical stated in slightly different ways. It’s crucial to be able to distinguish between isomers and analogous representations of the same substance. 1,2-dichloroethane, for example, can be expressed in a variety of ways.
Cis And Trans 1,2-Dichloroethane
1,1-dichloroethane is the isomer of 1,2-dichloroethane. The two chlorine atoms are bound to the same carbon atom in 1,1-dichloroethane, whereas they are bonded to distinct carbon atoms in 1,2-dichloroethane. The distinct condensed structural formulae, CHCl2CH3 and CH2ClCH2Cl, also reveal that two chlorine atoms are bonded to the same carbon in the first case, and two chlorine atoms are bound to neighbouring carbons in the second case.
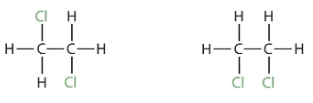
Restricted rotation around the double bond in 1,2-dichloroethene, on the other hand, means that the relative positions of substituent groups above and below the double bond become relevant. This results in a unique type of isomerism. The cis isomer (Latin cis, meaning “on this side”) of cis-1,2-dichloroethene is the isomer in which the two chlorine (Cl) atoms are on the same side of the molecule. The trans isomer (Latin trans, meaning “across”) has two Cl atoms on opposite sides of the molecule and is known as trans-1,2-dichloroethene. Because of the presence of a hard structure in their molecule, these two compounds are cis-trans isomers (or geometric isomers), which have different configurations (groups permanently in different places in space).
Key Points
- Ethylene dichloride, or EDC, is a kind of ethylene.
- It is mainly used in the production of PVC and is a highly flammable colourless liquid with a chloroform-like odour.
- It may be released into the environment during its manufacture or use.
- The general public may be exposed to very low levels of 1,2-dichloroethane as a contaminant in air or water. Inhaling 1,2-dichloroethane vapours can irritate the nose, throat, and lungs.
- Inhalation, ingestion, or skin exposure can produce initial excitation, headache, dizziness, sleepiness, cardiac issues, liver and kidney damage, and coma, among other things.
- Human cancer could be caused by 1,2-dichloroethane.
Conclusion
1,2-Dichloroethane, commonly known as ethylene dichloride, is a synthetic compound that does not occur naturally in nature. It’s a clear liquid with a lovely aroma and a sweet flavour. Vinyl chloride, which is used to create a variety of plastic and vinyl items such as polyvinyl chloride (PVC) pipes, furniture and automotive upholstery, wall coverings, kitchenware, and automobile parts, is the most prevalent usage of 1,2-dichloroethane.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




