Because of the presence of pi-electrons that are not tightly bound, alkynes undergo addition reactions. Because alkynes include a triple bond, other elements such as halogens, water, and other substances can be added to them by the addition reaction. A series of actions is required to create addition products. The production of addition products is attributed to the stability of vinylic cations in the presence of water. Asymmetric alkynes must adhere to Markovnikov’s rule in order to undergo an addition reaction, which they do by following the rule. The following are some examples of addition reactions using alkynes:
The Alkyne Triple Bond is a type of chemical bond.
The carbon-carbon triple bonds formed by two sp hybridised carbon atoms are formed by the overlap of orbitals on their respective carbon atoms.The molecule acetylene (C2H2) is claimed to have three sigma bonds and two pi bonds, according to the literature. The overlap of a sp hybrid orbital from each of the carbon atoms results in the formation of the C-C sigma bond in acetylene. The overlap of the second sp orbital on each carbon atom with the 1s orbital from a hydrogen atom results in the formation of the two C-H sigma bonds. Each carbon atom still has two half-filled p orbitals, which are perpendicular to each other and to the line created by the sigma bonds, and which are perpendicular to the line formed by the sigma bonds. As a result of these two perpendicular pairs of p orbitals forming two pi bonds between the carbons, a total of three bonds are formed between the carbons (one sigma bond plus two pi bonds). A negative belt (shown in red) is formed around the core of the molecule, as demonstrated by the electrostatic potential map of acetylene. The pi electrons of the triple bond form this belt. In accordance with VSEPR predictions, acetylene has a linear structure, with all four of its atoms arranged in a straight line and both H-C-C bond angles equal to 180o. Among carbon-carbon bonds, the triple bond formed by the element acetylene is the shortest (120 pm) and the strongest (964 kJ/mol) ever discovered.
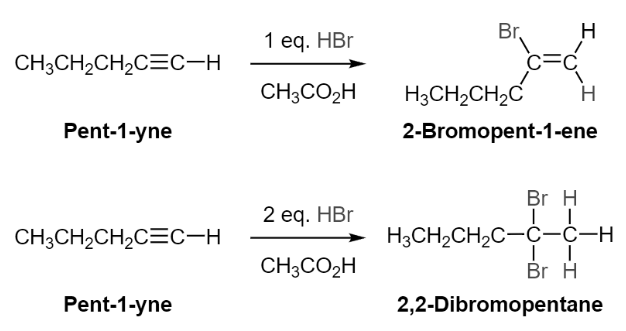
Addition of HX to Alkynes via Electrophilic Addition
Alkynes are subjected to electrophilic addition in a way similar to that of alkenes; however, the existence of two pi bonds makes it possible for the addition to occur twice in the same compound. Alkynes are converted to haloalkanes by the addition of one equivalent of hydrogen chloride or hydrogen bromide to the mixture. The addition of two or more equivalents of HCl or HBr to alkynes results in the formation of geminal dihalides via the formation of a haloalkene intermediary. They are regioselective and obey the Markovnikov’s rule in their placement. Although not usually the case, the double bonds generated during the reaction with internal alkynes have a Z stereochemistry, although not always.
Mechanism
The electrophilic addition of HX to an alkyne follows a mechanism that is similar to that of the electrophilic addition of HX to an alkene. Due to the presence of two pi bonds in the alkyne, the addition of HX can occur twice throughout the reaction. Following Markovnikov’s rule, the addition of H+ to the alkyne results in the formation of a vinyl cation, which will preferably form on the more substituted side of the alkyne. The subsequent addition of Br- results in the formation of a haloalkene, which then undergoes electrophilic addition to yield a second H+. The carbocation form will most likely form on the carbon that is already bonded to the halogen that is already there. Through the formation of a resonance structure that adheres to the law of octet, the halogen is able to maintain the stability of the carbocation. This stabilising effect ensures that just a geminal-dihalide is generated and that no vicinal-dihalide is formed as a result of the reaction.
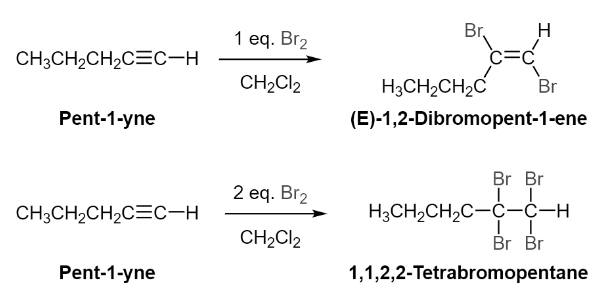
Addition of X2 to Alkynes via Electrophilic Addition
As with alkenes, alkynes are subjected to the same type of electrophilic addition with chloride and bromine. With alkynes, on the other hand, the halogen addition can occur once or twice, depending on the molar equivalents of halogen utilised in the reaction and the nature of the reaction. When one molar equivalent of halogen is utilised, the result is the formation of a dihaloalkene. Because of the anti-addition nature of the reaction process, the halogens in the resultant alkene are trans-converted. The addition of two or more molar equivalents of halogen causes the alkyne to be converted into a tetra haloalkane, which is then converted into a dihaloalkane.
Mechanism
The electrophilic addition of the alkyne with bromine results in the formation of a bromonium ion in a three-membered ring. The ejected bromide ion reacts with the bromonium ion in an SN2 reaction, causing the ring to open and the bromines in the resultant alkene to be in a trans configuration, as seen in the diagram. With the addition of a second pi bond, the method yields a tetrahaloalkane as a result of the resultant reaction.
In terms of electrophilic reagents, the relative reactivity of alkynes and alkenes is determined.
When the addition reactions of electrophilic reagents, such as strong Bronstead acids and halogens, to alkynes are investigated, a puzzling paradox emerges from the findings. The reactions of alkynes are even more exothermic than the additions to alkenes, despite the fact that the rate of addition to alkynes is a factor of 100 to 1000 slower than the rate of addition to alkenes. It can be seen in action in a reaction between one equivalent of bromine and 1-penten-4-yne, which results in the production of 4,5-dibromopent-1-yne as the primary product.
When alkynes react with electrophilic reagents, the processes are far more sluggish than when the analogous reactions occur with alkenes. Typically, addition reactions to alkynes are more exothermic than addition reactions to alkenes, and it would appear that there is a higher -electron density around the triple bond (two vs one -bond) in the presence of the triple bond. There are two key variables that contribute to the explanation of this apparent conundrum. Due to the presence of more -electrons in the triple bond, the sp-hybridized alkyne carbons are more electronegative than the sp2-hybridized alkene carbons in the first place. Due to the high attraction between the alkyne carbons and their -electrons, the alkyne carbons are more closely associated with their functional group than the -electrons of a double bond. This can be seen in the ionisation potentials of ethylene and acetylene, among other measurements. In the case of compounds, an ionisation potential refers to the smallest amount of energy required to remove an electron from one of the compounds’ molecules. Because the initial interaction between an electrophile and an alkene or alkyne involves the donation of electrons, the considerably slower reactions of alkynes can be explained by the fact that the electrophile donates electrons.
Conclusion
The primary reaction of alkynes is addition across the triple bond, which results in the formation of alkanes. Similar to the addition reactions of alkenes, these addition reactions occur in the presence of alkenes. Hydrogenation. Alkynes are hydrogenated catalytically using the same catalysts as alkenes: platinum, palladium, nickel, and rhodium. Platinum, palladium, nickel, and rhodium are the catalysts used in alkene hydrogenation. Hydrogenation occurs in a stepwise process, beginning with the formation of an alkene, which then undergoes further hydrogenation to generate an alkane.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




